Antithrombotics
Warfarin
Half-Life: 35-45 hours (Acenocoumarol 10 hours, Phenindione 8 hours)
Bleeding requiring hospital admission (% per year):
Aspirin 2.6%
Warfarin 4.3%
Clopidogrel 4.6%
Warfarin + Aspirin 5.1%
Warfarin + Clopidogrel 12.3%
All 3 taken together 12%
CYP2C9 mutation: Increases warfarin half-life and leads to over-anticoagulation
VKORC1 gene: Decreases warfarin requirements and leads to over-anticoagulation
Target INR
Management of High INR
Major Bleeding PCC + 5mg Vit K IV
Non-Major Bleeding 1-3mg Vit K IV & investigate source of bleeding
INR >8 not bleeding 1-5mg Vit K PO
INR 5-8, not bleeding Withold 2 doses and reduce maintenance dose
What constitutes poor INR Control?
Any one of:
Two INR values <1.5 in last 6 months
Two INR values >5, or One INR Value >8 in the last 6 months
Time in Therapeutic Range (TTR) less than 65%
CYP450 Drugs
Warfarin Teratogenicity (FWS)
Pathophysiology
Osteocalcin carboxylation for bone formation is a vitamin K dependent process
Warfarin has low molecular weight —> easy transfer across the placenta —> foetus more anticoagulated than the mother due to immature foetal liver enzymes
Discontinuing warfarin for pregnancy
Take pregnancy test at first day of missed period, with goal of stopping warfarin prior to 6 weeks.
First Trimester - Foetal Warfarin Syndrome (FWS)
Occurs following warfarin exposure during gestational weeks 6-12
Affects ~10% of foetuses exposed. Possible dose-response relationship.
6% congenital abnormalities
Nasal hypoplasia —> neonatal respiratory distress
Stipling of vertebrae & epiphyses —> hypoplasia of extremities
Second and third trimester risks
Risk of foetal, placental and neonatal haemorrhage
Other reported adverse outcomes from warfarin use in pregnancy
CNS – microcephaly, hydrocephalus, Dandy-Walker malformation
Eyes – blindness
Still birth, neonatal death, premature delivery
Others – scoliosis, developmental delay, deafness, CHD, seizures
Breastfeeding
Warfarin is safe in breast feeding
Warfarin & Anti-platelets
Already on anti-platelet and need to start warfarin
If on single anti-platelet <12 months after ACS should continue (consider using aspirin >clopidogrel)
If on DAPT, discuss with cardiology ?shorter duration
If on anti-platelet for 1o prophylaxis, PVD, prev stroke or stable IHD then stop the anti-platelet
Already on warfarin and need to start anti-platelets
If requires coronary stent, consider using bare metal stent
If PCI not required, give DAPT for 4 weeks then stop cloidogrel
Review the indication for warfarin and assess risk/benefit
Perioperative Management
Elective surgery
Stop 5 days prior to surgery, check INR day -1, give vit k if INR >1.5, check INR day of surgery
Restart at normal dose, or 2 days of double dose, on the evening of surgery
Bridging
Meta-analysis found increased bleeding rate with no reduction in thrombotic risk.
However, consider if:
<3 months since VTE and surgery cannot be delayed
High risk VTE patient – e.g. prev VTE whilst on anticoagulation
Mechanical heart valves except bileaflet aortic
<3 months since stroke/TIA
History of stroke/TIA plus 3 of: CCF, uncontrolled HTN, >75yo, Diabetes
Post-op - Use prophylactic dose, restart bridging at 48 hours.
Patient Self-Test and Self-Management
Available devices – CoaguCheck, Protime, INRatio
Quality Assurance
Internal QA – electronic, on-board (control integrated onto test strip), liquid control with INR of 2-4
External QA – every 6 months, test same sample on patient device and anticoag clinic equipment
Clinical outcomes – Reduced mortality and VTE. Unchanged bleeding rate. Trial included 99 patients >85 y.o.
Patient selection – Motivated patient expected to need AC for >1 year and successfully competes training.
Training consists of Theory and Practical aspects.
Wells Score for PE
Clinical signs and symptoms of DVT +3
PE is No. 1 diagnosis or equally likely +3
HR >100 +1.5
3 days immobile or surgery in last 4 weeks +1.5
Prev PE or DVT +1.5
Haemoptysis +1
Active Malignancy or treatment for malignancy in last 6 months +1
0-4 (12% incidence of PE) = Unlikely. Check D-dimer. If negative no further action.
5+ = Likely. Perform CTPA
Duration of Treatment for VTE
Initial
3 months for PE and proximal DVT
6 weeks for calf-vein DVT (or no treatment)
6 months LMWH (?or edoxaban) for cancer-associated VTE
Continued beyond 3 months:
Not required for VTE: provoked by surgery, non-surgical transient risks or confined to the calf
Consider long-term treatment:
Unprovoked VTE taking into account individual patient risks
See DASH Score
PE 3-4x more likely to recur than DVT and 2-4x more likely to be fatal if symptomatic compared to a symptomatic DVT
DASH Score
D-dimer abnormal 1 months after stopping anticoagulation +2
Age under 50 years old +1
Male Sex +1
Hormone use at time of VTE -2
0 = 2.4% annual risk of recurrence
1 = 3.9% 1 or less: consider discontinuing
2 = 6.3% 2 or more: consider LT anticoagulation
3 = 10.8%
4 = 20%
N.B. Score is based on meta-analysis of prev studies (warfarin only). It is not externally validated. Designed for use in stable patients with a first unprovoked VTE. Five-year recurrence of unprovoked VTE for all comers is 25-30%.
HAS-BLED (2010)
Predicts 1 year risk of major bleeding after starting anticoagulation. One point each for:
Hypertension
Abnormal liver
(& Abnormal renal function)
Stroke history
Bleeding predisposition
Labile INR
Elderly
Drugs that also risk bleeding (aspirin etc)
(& Alcohol)
Score 0-1 = Low risk (1-3%)
Score 2 = Moderate risk (4%)
Score 3+ = High risk (>5%), consider alternatives to anticoagulation.
Unfractionated Heparin
Derived from pig intestine.
Approx 1/3 of heparin molecules carry a high affinity polysaccharide that binds to Antithrombin (AT).
High Molecular Weight (HMW) Heparin-AT complexes then bind to thrombin (Why only the HMW chains? Because the heparin chain must be of sufficient length (molecular weight) to bridge between AT and thrombin).
Heparin-AT-Thrombin complex inactivates thrombin, preventing fibrin formation and inhibiting thrombin-induced activation of platelets, FV and FVIII.
Non-complexed heparin molecules and Heparin-AT complexes of any molecular weight can inhibit free FXa.
Half-life: 45-90 min (Variable due to binding to other positively charged plasma proteins & surfaces)
Molecular weight: 3,000 – 30,000 Da
Xa:IIa activity ratio: 1
Drug monitoring: Only use APTT ratio if pre-treatment APTT is normal. Reagents highly variable. Alternatives include: Anti-Xa, ACT and TEG
Emergency reversal:
Protamine sulphate
Dose: 1mg neutralizes 80-100 units of heparin, given IV over 5 minutes. Max dose 50mg.
Calculate dose based on previous 2 hours exposure. E.g. IV UFH 1250u/hour —> Give 25mg protamine
Short half-life, repeat doses may be required
Forms a stable, inactive salt with the heparin
Derived from fish sperm --> SE: Anaphylaxis. Risk increase with repeat exposure, rapid admin, and vasectomy
Low Molecular Weight Heparin & Heparinoids
Half-life: Approx 4 hours
Molecular weight: 1,000 – 10,000 Da (Mean: 5000)
Xa:IIa activity ratio: 2.5 – 4 (depending on preparation)
Target therapeutic Anti-Xa level:
4-6 hour peak = 0.5-1.0 iu/ml.
Pre-dose trough = <0.3 iu/ml
Produced from heparin by chemical or enzyme depolymerisation --> fragments approx. 1/3 the size of heparin
Results in reduced ability to bind thrombin for reasons given above
Reduced binding to other plasma proteins
Benefits Vs UFH:
More predictable dose-response relationship
Longer half-life
Reduced risk of HIT
Emergency reversal
Protamine reverse 60% of LMWH in healthy vounteers
Give only if last LMWH dose within 8 hours of bleeding event
Dose: 1mg per 100 Anti-Xa units of LMWH. Repeat dose with 0.5mg per 100 units if required.
Consider rFVIIa if life-threatening bleeding despite the above and timeframe consistent with ongoing LMWH effect
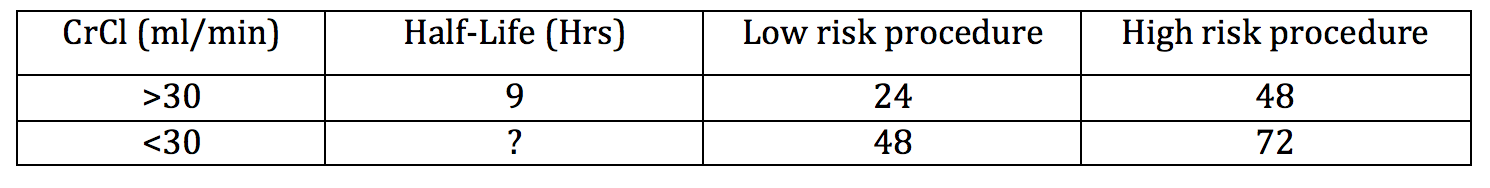
Fondaparinux
Half-life: 17-21 hours
Synthetic pentasaccharide, Xa inhibitor
rFVIIa in life-threatening bleeding (causes partial correction in healthy volunteers
Danaparoid
Heparinoid, derived from a different fraction of porcine bioproducts
Half-life: 25 hours (Xa), 7 hours (Thrombin)
Xa:IIa activity ratio: 20
Plasmapheresis will remove danaparoid from circulation.
DOAC vs VKA Overview
DOAC Limitations
Short half-life and lack of monitoring —> potential compliance issues
Limited lab assessment in emergencies
Limited used in chronic renal impairment
When to maybe measure DOAC levels
Spontaneous or traumatic haemorrhage
Following overdose or to assess compliance
When take interacting drug
Emergency surgery, neuroaxial anaesthesia and elective surgery
Renal impairment
Bridging from one AC to another
Extremes of weight
Trough levels for accumulation in the very elderly
Warfarin still best for:
Mechanical Heart valves
Mitral Stenosis
Antiphospholipid syndrome
Long-term stable INR and patient choice
Poor renal function
History of GI bleed
Questionable compliance
Drug interactions that prevent use of DOAC
AC in ‘Non-Valvular’ AF (2017, 2016)
DOAC trials varied in how broad their definition of NVAF was in the exclusion criteria
Native valve disease
CHA2DS2VASc score 2 or more, plus aortic valve disease, tricuspid disease or MR
Can be considered for a DOAC
Moderate to severe mitral stenosis
Continue to use VKA only
Bioprostheses
Can be considered for DOAC after the third month since implantation (ESC)
(N.B. for TAVI patients without AF, dual antiplatelets are used, not AC. Trials underway for DOACs)
Mechanical prostheses
Continue to use VKA only
Dabigatran Etexilate
Half-life: 13-18 hours (see below)
Dose: 150mg BD (110mg BD if age >80 or >75 plus high bleeding risk)
Mechanism:
Pro-drug --> absorption is pH sensitive --> reduced absorption with use of PPI
Reversible, high affinity binding to thrombin
Direct Thrombin Inhibitor is a reference to heparin, i.e. anticoagulant effect is independent of antithrombin
Coag Assays:
PT normal in 30% of patients
APTT usually prolonged – rate of prolongation flattens out as drug concentration increases —> insensitive
TT very sensitive – at high concentration will be prolonged beyond a detectable time. If the TT is normal, patient is not taking dabigatran.
Haemclot Assay – a modified TT assay designed for dabigatran monitoring
Chromogenic Anti-IIa assay also available
ECT – directly assays thrombin activity in plasma —> linear dose-response to therapeutic doses of dabigatran
Drug levels: For 150mg BD, expected peak = 0.184mg/l and trough = 0.09mg/l
Factor assays and FGN all underestimated in presence of dabigatran if clot-based assays used.
DRVVT false positives occur
Perioperative table
80% renally excreted, independent of CYP450 enzymes
Re-start at full dose 6-12 hours after minor procedure, or 48-72 hours after major surgery.
Drug Interactions
P-gp pathway affects absorption of the exetilate pro-drug
Inhibitors increase plasma drug concentrations
Azoles, amiodarone, diltiazem, tacrolimus
Inducers reduce plasma drug concentrations
Rifampicin, St Johns Wort, Carbamazepine
Reversal
Activated charcoal if last dose within 2 hours prevents further absorption
Idarucizumab
Monoclonal antibody fragment. Rapid clearance. Licensed 2016.
Dose: 5g, given as 2x 2.5g bolus infusions within 15 minutes of each other.
Trials (“RE-“ studies)
AF: RE-LY
VTE Treatment: RE-COVER & RE-COVER2
VTE 2o prevention: RE-SOLVE & RE-MEDY
VTE LT 2o prevention: RE-SONATE
ACS: RE-DEEM
TKR prophylaxis: RE-MODEL & RE-MOBILISE
THR propjhylaxis: RE-NOVATE
Other Direct Thrombin Inhibitors
Argatroban
Half-life: 50 minutes
IV infusion, licensed for treatment of HIT
Monitor with APTT ratio (aim 1.5 – 3.0)
Rapid hepatic clearance via CYP450 3A4 enzyme
Hirudin
Natural peptide from Hirudo medicinalis (medical leech)
10% of patients develop anti-hirudin antibodies à actually increases half-life
Therapeutic drug concentration: 0.5-2.5 microg/ml
Monitoring: APTT, ECT, TT, ELISA, Chromogenic, Haemolclot
Lepirudin
Half-life: 1.3 hours
Continuous IV/SC infusion
Recombinant hirudin
Bivalirudin
Half-life: 25 minutes
Short, synthetic peptide. Binds to circulating and clot-bound thrombin
Reversible as thrombin slowly cleaves the drug (accounts for 80% of drug clearance. 20% renal)
Monitor with ACT during cardiac surgery
Factor Xa Inhibitors
Coag Assays:
PT usually more prolonged than APTT (Rivarox > Apix) but both may be normal
Monitor with product-specific Anti-Xa chromogenic assay
TT, FGN and D-dimer unaffected
DRVVT false positives
Drug Interactions:
CYP3A4 and P-gp inhibitors both increase rivaroxaban concentrations
Ketaconazole, Ritonavir
Reversal:
Prothrombin complex concentrate (PCC)
Octaplex 30 units/kg (max 3000 units) / Beriplex 50 units/kg (max 5000 units)
This is a supportive measure rather than a reversal / antidote to the DOAC effect
Andexanet alfa
Recombinant modified human factor Xa decoy protein. ~£15,000 per treatment.
ANNEXA-4 2019. Single arm trial. 1o outcomes were change in anti-Xa activity and physician-assessed haemostatic efficiency.
Given as a bolus followed by infusion.
Approved by NICE in 2021 solely for use in the setting of life-threatening GI bleeding (and only for rivaroxaban or apixaban. Edoxaban is not included in the NICE recommendation)
ANNEXA-I. 530 adults with intracranial haemorrhage on rivaroxaban or apixaban. Andexanet vs Standard Care (87% of which got PCC). Study stopped early at interim analysis, due to superiority in primary outcome (‘effective haemostasis at one month’) vs usual care. No diff. in functional outcome or 30-day mortality. higher rate of thrombosis in andexanet arm. Written publication awaited (as of Oct 2023)
As of 2023, there has been no direct comparison of andexanet vs PCC
Perioperative:
Pre-op: See table for each drug
Post-op: Re-start at full dose 6-12 hours after minor procedure, or 48-72 hours after major surgery.
Rivaroxaban
Half-life: 7-11 hours
Dose: For treatment of VTE = 15mg BD for 21 days, then 20mg OD
Expected peak PT ratio = 1.3-16 and trough = normal
Trials: ROCKET-AF, EINSTEIN, RECORD, SELECT-D
Apixaban
Appears to have lower rate of bleeding complications compared to other DOACs (e.g. this and this) with the important caveat that there are no head to head trials to draw this conclusion from.
Half-life: 9-14 hours
Dose for treatment of VTE: 10mg BD for 7 days, then 5mg BD, then 2.5mg BD after 6 months if ongoing AC
For 5mg BD, expected peak = 0.128mg/l and trough = 0.05mg/l
Trials: ARISTOTLE, Caravaggio, ADAM
Edoxaban
Lactose free (unlike other Xa inhibitors)
Half-life: 10-14 hours
Dose for treatment of VTE: 5 days LMWH, followed by 60mg OD (30mg for CrCl 15-50, wt <60kg, interacting meds)
Trials: Hokusai-VTE (Non-inferiority to dalteparin for treatment of VTE in cancer)
Antiplatelet Agents
Aspirin
Mechanism:
Irreversible binding to cyclooxygenase (COX1)
--> prevents thromboxane A2 generation and so prevents plt aggregation
As platelets are non-nucleated they cannot produce new COX1
--> drug effect 7-10 days (lifespan of platelets)
Perioperative
Associated with 1.5-fold increase in post-op bleeding but not in the severity of the bleed
Continue through most surgery – exceptions: neuro and prostate surgeries
Management of Emergency surgery with high bleed risk:
Consider 2 units platelets >2 hours since last dose (4-5 hours if enteric coated)
(aspirin-inactivated platelets can still be recruited by thromboxane generated in the transfused platelets)
Half-life: 3 hours
Onset of action: 1 hour
Time from drug admin during which platelet transfusion will have reduced efficacy: 2 hours
Time to normal platelet function after discontinuation: 5-7 days
Clopidogrel
Mechanism:
Binds to P2Y12 --> preventing ADP-induced plt aggregation
(Normal action of ADP is to bind to P2Y12 and P2Y1, in turn activating Gp IIb/IIIa)
Metabolised by CYP2C19
Defective genotypes vary by ethnicity and can cause clopidogrel refractoriness
Perioperative
Emergency surgery with high bleed risk
Consider 2-4 units platelets >12-24 hours since last dose (based on little evidence)
Stop day -7 prior to neuroaxial procedures
Half-life: 6 hours
Onset of action: 3-4 hours
Time from drug admin during which platelet transfusion will have reduced efficacy: 12-24 hours
Time to normal platelet function after discontinuation: 5-7 days
Dual Antiplatelet Therapy (ESC 2017)
Typical DAPT treatment durations
Minimum 4 weeks post bare metal
Minimum 12 months post drug-eluting
<12 months for newer bioabsorbable drug-eluting
May differ for high bleeding risk patients (e.g. NEJM 2021 - no difference 1 vs 3 months DAPT following drug eluting stent)
Thrombotic risk categories for patients on DAPT
Very high: ACS <8 days, drug eluting stent <8 days
High: ACS 8-30 days, drug eluting stent 8-30 days
Moderate: ACS 1-12 months, drug eluting stent 1-12 months
Low: Cerebrovascular and peripheral vascular disease, ACS >12 months, drug eluting stent >12 months
Perioperative
4-8% of patients need surgery within 12 months of starting DAPT
Hold clopidogrel from day -5, continue aspirin for most low risk procedures, inc neuroaxial ones
High bleeding risk, also omit aspirin day -3 to day +7
If high risk surgery cannot be deferred in a patient with recent ACS
Continue aspirin, stop clopidogrel/ticagrelor day -5 or prasugrel day -7
For high thrombotic + high bleed risk CABG patients
Stop antiplatelets, consider parenteral GpIIb/IIIa inhibitor day -3 to 4-6 hours post op
Peri-Procedure
Lumbar puncture: Do not perform whilst on DAPT. If clinically justified then performing LP can be considered whilst on aspirin alone.
Gastro procedures: BSG 2021 guideline
Bleeding (see pages 246-248 ESC guideline for flowchart):
Weigh bleeding severity against thrombotic risk (above)
Trivial bleeding: Continue DAPT
Mild bleeding (No hospitalisation): Consider reducing duration of DAPT
Moderate bleeding (hospitalisation, no CVS compromise): Stop DAPT, continue single agent
Severe (Major blood loss): Stop DAPT, treat source of bleeding, continue single agent unless bleeding source cannot be controlled
Life-threatening: Stop anti-platelets, treat source of bleeding
Trial data to consider:
ATACAS 2017: TXA vs placebo in cardiac surgery. No increase in thrombotic complications
PATCH 2016: Non-traumatic ICH on anti-platelets, not fit for urgent surgery. Platelet transfusion vs standard of care. Primary outcome: increased mortality/disability at 3 months in platelet arm. Surprising finding, lead to further analysis in 2020 but still no evidence for platelet benefit.
Zakko et al 2017: case-control trial. Platelets for GI bleeding on anti-platelets. No benefit but also no harm.
RESTART 2019: Re-starting anti-platelets after ICH. Small trial. Larger trials in progress.
Prasugrel
Thienopyridine drug class (same as clopidogrel)
Mechanism:
Binds to P2Y12 preventing ADP-induced platelet aggregation
Prodrug. Converted to active drug by CYP450. Metabolised more efficiently than clopidogrel resulting in a greater antiplatelet effect.
Perioperative
Emergency surgery with high bleed risk
Consider 2-4 units platelets >12-24 hours since last dose (based on little evidence)
Half-life: 7 hours
Onset of action: 2-4 hours
Time from drug admin during which platelet transfusion will have reduced efficacy: 16-18 hours
Time to normal platelet function after discontinuation: 5-7 days
Dipyridamole
Mechanism:
Inhibits phosphodiesterase --> accumulation of cAMP --> Intracellular Ca2+ falls --> blocks response to ADP --> reduced thromboxane A2 production preventing plt aggregation.
Also prevents cellular uptake of adenosine
Alpha half-life: 40 min, Beta half-life: 10 hours
Onset of action: 1 hour
Time from drug admin during which platelet transfusion will have reduced efficacy: 5-7 hours
Time to normal platelet function after discontinuation: 24 hours
GPIIb/IIIa Inhibitors
Abciximab
Mechanism
Human-murine antibody to GpIIb/IIIa, close to FGN binding site
SE: Thrombocytopenia in 0.5%. Onset in hours to days. Risk increases with repeated exposure
Half-life: 30 minutes
Time from drug admin during which platelet transfusion will have reduced efficacy: 1-2 hours
Time to normal platelet function after discontinuation: 24-48 hours
Tirofibran
SE: Thrombocytopenia in 0.5%
Half-life 2 hours
Time from drug admin during which platelet transfusion will have reduced efficacy: 4 hours
Time to normal platelet function after discontinuation: 4-8 hours
Eptifibatide
Half-life 2.5 hours
Time from drug admin during which platelet transfusion will have reduced efficacy: 4 hours
Time to normal platelet function after discontinuation: 4-8 hours
Significant Others
Ticagrelor
Mechanism
Reversible binding of ADP receptors
50-60% inhibition of ADP-induced plt aggregation (>clopidogrel)
Perioperative
Emergency surgery with high bleed risk
Consider 4 units platelets >24 hours since last dose (based on little evidence)
Half-life: 6-13 hours
Onset of action: 1-2 hours
Time from drug admin during which platelet transfusion will have reduced efficacy: 18-26 hours
Time to normal platelet function after discontinuation: 3-5 days
Cangrelor
IV reversible ADP P2Y12 inhibitor
Continuous infusion
Half-life 90 min
Plt return to normal after 60 minutes
Vorapaxar & Atopaxar
Reversible PAR-1 inhibitors
PAR1 and PAR4 receptors are activated by thrombin leading to plt aggregation.
Fibrinolytics
Streptokinase
IV infusion
Enzyme produced by Group C haemolytic streptococcus
Binds to plasminogen activating it to plasmin independently of fibrin
Hyperfibrinolytic effect lasts a few hours after stopping infusion, but TT remains prolonged for 24 hours.
Tissue Plasminogen Activator (t-PA)
Human recombinant protein
Causes fibrinolysis only at the site of vascular injury
Reteplase
Non-glycosylated t-PA —> longer half-life
Tenecteplase
Modified t-PA —> longer half-life
Urokinase
Half-life: 12 minutes
IV infusion
Obtained from human neonatal kidney cells
Emergency reversal of Fibrinolytics
For cerebral bleeding within 48 hours of administration
FFP + TXA +/- FGN replacement