Mantle Cell Lymphoma (MCL) (BSH 2023)
t(11;14) --> overexpression of Cyclin D1--> deregulation of cell cycle at G1-S phase boundary
Immunohist: Cyclin D1+, BCL2+, SOX11+, BCL6-
Flow: CD19+, CD20+, CD5+, FMC7+, Surface Ig+, CD10-, CD23-, CD200-
Intro
3-10% of NHL
Median age 60-65 years old
Male > Female
Worst features of low and high grade lymphoma. Incurable by standard therapy
Median survival 4-5 years in all comers (8-12 years in younger, fitter patients) (5-yr OS 58% in this 2023 paper)
CNS relapse occurs in 4-8% - usually leptomeningeal and within 2 years
Clinical Presentation
>90% present with Stage 3-4 disease
Splenomegaly, bone marrow infiltration and leukaemic involvement are common
30-50% of patients have >2 extranodal sites involved – esp. GI and liver
Variable clinical course – aggressive blastoid variant or indolent disease
DIAGNOSIS
Sample
Excision or core biopsy, or peripheral blood flow if leukaemic
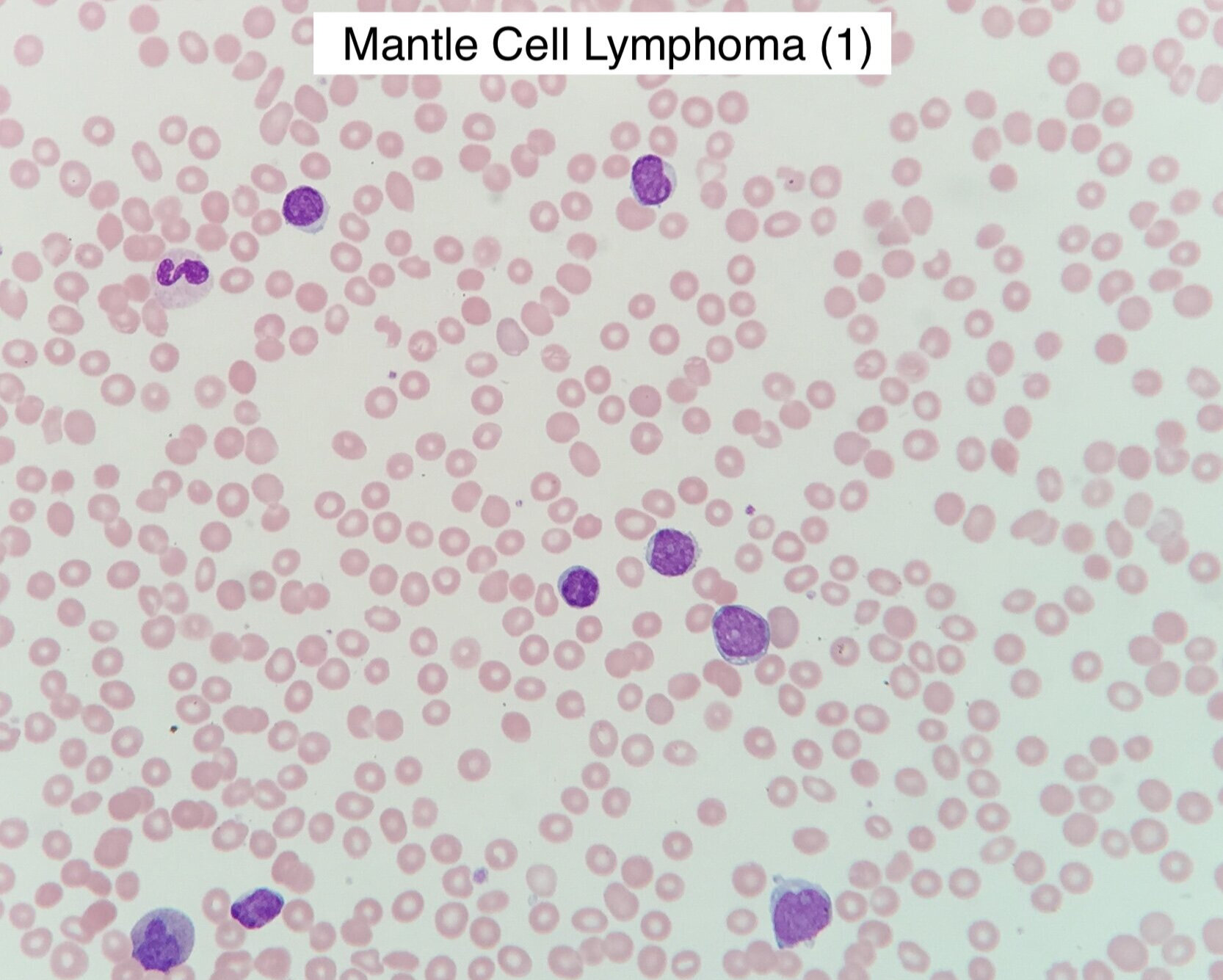
Peripheral Blood Morphology
Small to intermediate sized cells with irregular, cleaved nuclei
Larger atypical forms in blastoid variants
Immunophenotype
CD19+, CD20+, CD5+, CD79b+, CD22+, FMC7+, ROR1+, Surface Ig+ (usually lambda)
CD10-, CD23-, CD200-
Aberrant phenotypes – many variants including cyclin D1 neg, CD10 pos or CD5 neg
Histology
Variable - classic, blastoid, pleomoprhic, marginal zone-like and small cell types described
Ki67 proliferation index is a prognostic factor (>30% correlates w/ poor outcomes)
Immunohisto: Cyclin D1+, often SOX11+ (more details in guideline)
Cytogenetics
t(11;14)(q13;q32) (—> Cyclin D1 overexpression) can be detected by FISH
If Cyclin D1 negative then molecular testing required
Additional features: loss of 1p, 6q, 8p, 9p, 10p, 11q, 13p, 17p
Additional features: gains of 7p, 3q, 8q, 12q, 18q
Molecular
ATM most frequent mutation
TP53 mutation strongest predictor of poor outcomes (treatment response, early progression, mortality)
Others: NOTCH1, CDKN2A, CCND1, NSD2, KMT2A, S1PR1, CARD11
CCND2 and CCND3 mutation testing for Cyclin D1 negative cases
MCL in situ
syn. Mantle Cell Neoplasia – CD5+, Cyclin D1+ small lymphocytes in the mantle zone of follicles in morphologically reactive lymph nodes. Low risk of progression to clinical disease.
Differential Diagnosis of Cyclin D1+ haem malignancies:
Hairy Cell Leukaemia
Myeloma
Diffuse Large B Cell Lymphoma
INVESTIGATIONS / work-up
Bloods
FBC, Film, Flow cytometry
U&E, LFT, LDH, Urate
HIV, Hep B/C
Staging
PET-CT preferred (superior to CT in detecting nodal and splenic invovlement)
BM involved in 50-90% of cases. Biopsy recommended as PET-CT has poor detection rates
GI tract involved in 15-30% of cases. Routine endoscopy unlikely to affect management. Consider OGD for patients otherwise thought to be early stage headed for radiotherapy.
CNS involvement incommon at diagnosis. MRI + CSF only if symptoms to indicate.
Frailty assessment
Frailty affects response and risks of intensive treatement
Consider use of “Geriatric 8” or “Geriatric Assessment in Haematology” tools
Fertility assessment
Discuss where relevant
Prognostic Scores
MIPI (Mantle Cell IPI), s-MIPI, MIPI-B and MIPI-C all provide prognostic information but do not currently affect treatment decisions. MIPI was predictive of OS and PFS in MCL2 trial.
MIPI Score
Uses age, ECOG PS, LDH ratio to ULN and WBC
MANAGEMENT
1st Line Management
Stage I/IIa
5% of cases. Limited evidence available.
Include endoscopy, BM biopsy and PET-CT in staging investigations to confirm early stage
Options: W&W versus localized involved field radiotherapy (60-80% CR w/ possible cure)
Stage III/IV, Indolent Disease
10-15% of cases
Typically non-nodal with IGHV-mutated and SOX11-negative or nodal with a low Ki-67
Can W&W closely, defers treatment by median of 12 months
Indications to treat include: Bulky LN, B symptoms, Symptomatic organomegaly, BM failure
Stage III/IV, Fit for autograft
Typically <65 yo
No specific induction regimen superior, but should include rituximab and high dose cytarabine
e.g. R-Maxi-CHOP/cytarabine (NORDIC Protocol) --> Autograft in 1st CR
e.g. R-DHAP x4 (LyMa Trial) --> Autograft in 1st CR
Autograft significantly prolongs PFS, not clear that it improves OS.
R-maintenance post autograft 2-monthly for 3 years
Proven benefit after R-CHOP / R-DHAP (4-year PFS 85% / OS 89% vs 64/80% w/out)
Addition of ibrutinib to induction and maintenance is beneficial but not currently available in UK (2023)
TP53 patients very poor outcomes with the above. Consider clinical trial to access alternative strategies, e.g. upfront CAR-T
Stage III/IV, Older/Less fit
Consider using “Geriatric 8” or “Geriatric Assessment in Haematology” to aid assessment
R-CHOP / R-Bendamustine / R-BAC / VR-CAP
R-maintenance 2-monthly until progression (funding restricts to a maximum 2 years)
Proven benefit post R-CHOP with prolonged PFS (and OS in some studies)
No benefit demonstrated post R-Benda in original trials. Subsequent real world evidence indicates a benefit and should be offered.
Assessing Treatment Response
Assess response with an end of treatment imaging - PET-CT or CT
Minimal residual disease (MRD) monitoring of t(11;14) by PCR may predict relapse but is not currently in routine use.
relapse/refractory management
Excluding transplant, 1st relapse has median survival of 1-2 years
Fit, high-risk patients should be considered for CAR-T
Re-biopsy for histological subtype, Ki-67 and TP53 mutations
Refer to CAR-T centre at start of BTKi
Close monitoring Re-image 8-12 weeks after starting BTKi
Ibrutinib
Approved for 2nd line use (NICE 2018)
Most active single agent in relapsed MCL – 68% response rate, 21% CR.
Also shown efficacy in CNS relapse
Other BTKi’s not currently available in UK (2023)
CAR-T
Brexucabtagene autoleucel (brexu-cel) - autogolous, CD19-targeting CAR-T
Approved for use after 2+ lines of treatment including a BTKi (NICE 2021)
If fit for CAR-T, pts should undergo interim imaging after 8-12 weeks of BTKi
Post-BTKi and Post/not-fit CAR-T
Consider clinical trial where possible
R-CHOP / R-Benda / R-BAC, whichever not used previously
Non-covalent BTK Inhibitors, pirtobrutinib, if available
Bortezomib, Temsirolimus & lenalidomide are all licensed for relapsed MCL
Venetoclax looks active in phase 1 trial
Allogenic Transplant?
Consider for fit patients following immunochemotherapy, BTKi and CAR-T
A few trial notes
870 patients <65 yo, untreated mantle cell lymphoma
Addition of ibrutinib to induction and maintenance
3 arms: Autograft, Autorgaft + Ibrutinib, Ibrutinib
Outcomes superior in ibrutinib arms - not currently available in UK for this indication (2023)
Phase 2, 74 patients, CAR-T for R/R mantle cell. Up to 5 prev therapies, including a BTKi
12 month PFS 61%
12 month OS 83%
299 patients, R-DHAPx4, followed by BEAM Autograft (R-CHOPx4 given is not in CR/PR post DHAP)
The R-DHAP could be with cisplatin or oxalaplatin
Post autograft, randomised to rituximab maintenance or not
4-year OS 78%, PFS 67% and superior in the R-maintenance arm
Phase 3, open label. 500 patients <65yo with untreated mantle cell
2 arms: R-CHOP x6 + Autograft versus R-CHOP/R-DHAP alternating x6 + Autograft
Median time to treatment failure: 9.1 yrs cytarabine vs 3.9 yrs without
Alternating cycles of R-Maxi-CHOP and High dose cytarabine for 6 cycles, followed by autograft
5-year EFS >60%
2016 update: No plateau in the survival curve, with ongoing late relapse events
2016 update: 40% of low and intermediate risk patients still in 1st remission at 12 years