Acute Lymphoblastic Leukaemia
(ESMO 2016, UKALL14, BCSH 2018)
B-ALL: TdT+, CD19+, CD10+/-, CD20+/-, cIg+/-, sIg +/-
T-ALL: TdT+/-, CD7+, CD2+, cCD3+, CD5+/-, sCD3+/-
Intro
Rare disease in adults.
1 per 100,000 per year in Europe
Risk Stratifications
High/Poor Risk
Age >40/55/65
WBC >30 (B-ALL) or >100 (T-ALL)
>4 weeks to reach CR
t(9;22) BCR-ABL1 (Philidelphia chromosome, “Ph+”)
t(1;19) PBX-E2A
t(4;11) MLL-AFA4
Hypodiploidy (e.g. del(6q), del(7p), del(17p), -7)
NOTCH1 unmutated
Complex Karyotype (5 or more clonal abnormalities)
Standard Risk
Not high risk
More favourable, but rare in adults, are t(12;21) TEL-AML1 and hyperdiploidy
Prognosis
UKALL 2012 five-year survival (patients recruited between 1996-2006)
Ph+ 22%
Ph- 43%
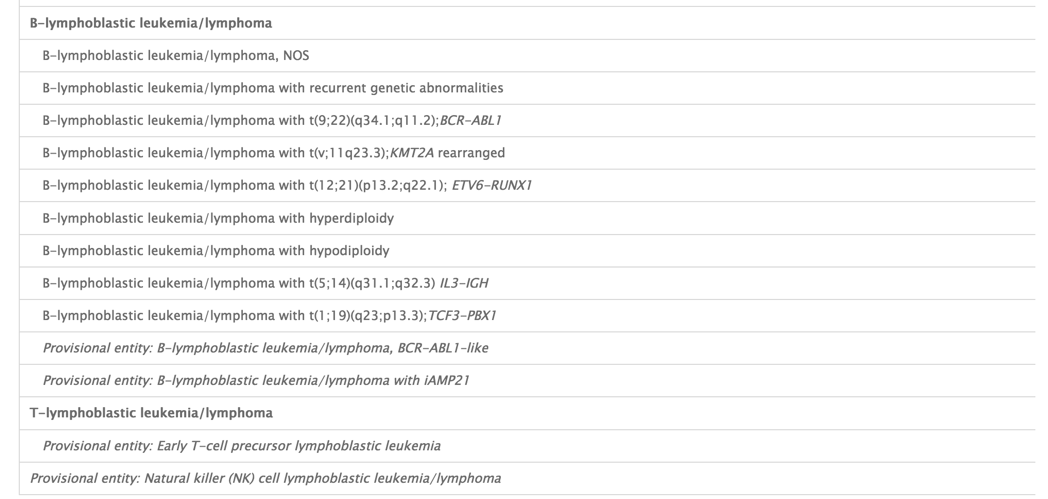
WHO 2016 Classification
investigation
Initial work up should be completed within 1-2 days to confirm diagnosis and prevent delay in Rx
CSF
For assessment of CNS involvement
BM Aspirate Morphology
>20% blasts, differentiates from lymphoblastic lymphoma with marrow involvement
Flow Cytometry
MPO negative, differentiates from AML
B-lineage
Pro-B: CD19+, CD79a+, cCD22+
Common: CD10+, cIg –
Pre-B: cIg+, sIg-
Mature: sIg+
T-Lineage
Pro: cCD3+, CD7+
Pre: CD2+, CD5+
Cortical: CD1a+
Mature: CD3+, CD1a -
Cytogenetics / FISH / RT-PCR for adverse features:
Rapid detection kit for t(9;22)
t(4;11)
t(1;19)
Next Generation Sequencing (NGS) for adverse features:
Ph-like ALL
ETP ALL
NOTCH1/FBW7-unmutated/RAS/PTEN-altered
IKZF1, CLRF2, MLL, TP53, CREBBP
Identification of a MRD marker
Flow or PCR
HLA Tissue Typing
Including parents and siblings
Cell banking
Future research
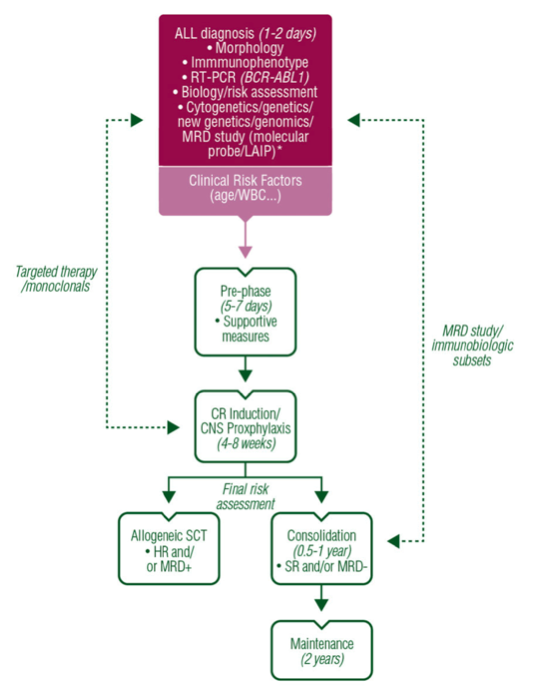
Treatment Principle (ESMO 2016)
Source: ESMO Guideline
Pre-Phase
Consist of:
Prednisolone 20-60mg/day or Dexamethasone 6-16mg/day
Hydration + Allopurinol
+/- Rasburicase
+/- Vincristine
Provides safe tumour reduction, usually avoiding TLS
Allows time for results of cytogenetics for risk stratification
Induction & Consolidation
Two broad approaches
- BFM (Berlin-Frankfurt-Munster) protocols, e.g. UKALL14
- Alternating two chemo regimens for 8 cycles, e.g. hyper-CVAD
UKALL14 Protocol
Treatment Rationale
Immunosuppressive > Myelosuppressive, but harder to tolerate
15-17 yo treated by paediatric regimens do significant better than those on the adult regimens --> Why?
Paediatric regimens are more immunosuppressive - prednisolone, asparaginase, vincristine
Adult regimens are more myelosuppressive – daunorubicin, cytarabine, cyclophosphamide
Dexamethasone > Prednisolone, Why? (Leukaemia 2011)
Better in vitro anti-leukaemic effect
Greater CNS penetration
Reduced CNS relapse rate (RR 0.5)
Reduced risk of death / relapse / 2o malignancy
At the cost of: 7x increase in myopathy, more neuro SE’s, more mid-treatment deaths
Vincristine
Inhibits microtubule formation
Neuropathic side effects worse in presence of CEP72 gene mutations
Consider TPMT status
Asparaginase
Derived from E.coli, Pegylated-E.coli or Erwinia
Unlike normal cells, leukaemic cells unable to produce asparagine —> Asparaginase depletes asparagine and starves leukaemic cells of amino acid essential for replication.
Adverse Effects
Thrombosis – depletes AT, Prot C and Prot S. Evidence of AT replacement is marginal
Immunogenicity – some patient develop antibodies against drug à rendered ineffective
Pancreatitis
Hepatic toxicity
Anthracyclines
Daunorubicin vs Doxorubicin – not much difference
Mitoxantrone > Idarubicin in treatment of relapse
CNS Prophylaxis
Combination of intrathecal / high dose intravenous methotrexate, steroids and cytarabine
Reduces CNS relapse rates from 10% to <5%
Historical use of craniospinal radiotherapy caused increased rates of 2o malignancy
Role of Stem Cell Transplant
Adults > Children, as cure rates so good in children
Adult relapse --> survival <10% with salvage chemotherapy
Limited evidence for Graft vs Leukaemia effect in ALL transplants
UKALL 2012 & Transplant
Biological randomization to transplant – i.e. all patients with a matched sibling got HSCT
53% patients had a matched, sibling HSCT
Increased treatment-related mortality upfront
Long term reduced relapse rate
No benefit from Autograft
Currently who to offer HSCT in 1st CR:
Improves OS and EFS for high risk, MRD+, Ph+ or MLL-rearranged patients
For Ph+ Patients post-allograft
Continue TKI maintenance
3 monthly IT/LP in patients who received reduced intensity conditioning (RIC)
Treatment Complications
Osteonecrosis
Not just steroid effect. Seen pre-treatment in acute leukaemia patients
Risk Factors
Age
Female
BMI >26
Drug causes:
Steroids, Aspraginase, MTX, Cyclophosphamide
Long-term
Endocrine (thyroid/gonad), Skin & mucosal disorders, Catarct, CVS disease, Infection, GVHD
Second malignancy (<3% of patients)
Other Therapies
Rituximab
CD20 present on 30-50% of pre-B ALL cells
Pre-treatment with steroids increases the expression of CD20
Better EFS and reduced relapse seen in GRAALL-R 2005
Blinatumumab
Bispecific antibody, CD19 + CD3 - Brings T-cells into contact with tumour target
Use in primary refractory and relapsed ALL
Pros: Good for low level disease (eg MRD+)
Cons: 28 day infusional treatment. Neurotoxicity. Theoretical concern re: losing CD19 as a target for subsequent CAR-T
Inotuzumab Ozogamin
Anti-CD22 monoclonal antibody bound to calichemicin
Promising role in elderly patients in combo with mini-hyper-CVAD
Pros: Avoids CD19 target, thinking of subsequent CAR-T use
Cons: Up to 20-30% increased risk of VOD in any subsequent allograft
Chimeric Antigen Receptor (CAR) T-Cells
NEJM 2014 – CTL019-transduced autologous T cells can induce sustained remissions
At present, allograft still probably comes first, CAR-T for further down the line. This may change.
SE: Severe cytokine release syndrome requiring ITU support
Relapse/Refractory disease
The following notes on R/R disease are based on local practice/teaching - treatment needs to be individualised and will be debateable
Frank refractory disease after Phase 1 induction
Alternative intensive induction, e.g. FLAG-Ida
If Ph+, check the BCR-ABL1 mutation, e.g. is a different TKI indicated?
Low level BCR-ABL1 MRD positivity after Phase 1 induction in Ph+
Consider proceeding straight to transplant (ie not chasing MRD negativity)
MRD positivity after Phase 2 induction in Ph neg B-ALL
Blinatunumab > Inotuzumab (see above in Other Therapies)
Relapse <12 months post allograft
Clinical trial
Inotuzumab re-induction prior to CAR-T
Relapse >12 months post allograft
Clinical trial
Inotuzumab —> CAR-T —> Consider 2nd Allograft
Children
Outcomes
Cure rates now approx. 90%
Therefore risk stratification ever more important to reduce Rx-related morbidity
Risk Stratification
1966 Sidney Fisher
‘not possible to predict outcome’
1990’s Clinical Risk
10 or more years old & WBC >50 poor risk in B-ALL
Currently
Clinical features at diagnosis
Age <1 or >10 (Age correlates with cytogenetic findings)
WBC >50
CNS disease
Down Syndrome
Male
Black/Hispanic
Disease Characteristics
T-ALL
Hyperdiploidy
TP53 mutation
Ph+
MLL, RUNX1, Ph-like ALL, IKZF1
Response to initial therapy
BM at day 8 (Regimen B) or day 15 (Regimen A)
<25% = rapid early response
>25% = slow early response
Minimal Residual Disease at day 29 (UKALL11)
Flow vs PCR
83% of relapses in UKALL 2003 were in MRD+ patients
Treatment (UKALL 2011)
Pre-B ALL with NCI standard risk (Age <10, WBC <50)
Regimen A (3 drugs)
Pre-B ALL with NCI high risk or T-ALL or Lymphoblastic Lymphoma
Regimen B (4 drugs)
If meeting certain criteria for poor risk features as treatment progresses
Switch from Regimen A/B to Regimen C
Treatment split into:
Induction
BFM consolidation
Interim maintenance
Delayed Intensification
Maintenance
Total 2 years for girls, 3 years for boys (due to testicles as sanctuary site risk)
Different nomenclature to the adult UKALL14 but an essentially similar strategy
BSH 2018: Management of thrombotic and haemostatic issues in paediatric malignancy
VTE
Incidence
Reports vary widely
Asymptomatic thromboses identified by radiological screening in up to 40% of patients
More common in ALL, Sarcoma and Lymphoma.
Risk Factors
Patient-related
Age >10
Inherited thrombophilia
Personal or family history of VTE
Obesity
Immobilisation
Concurrent infection
Disease-related
Pulmonary/intrathoracic/pelvic disease
Sarcomas
APML
ALL
Lymphoma
Treatment-Related
Major surgery
Central lines
Induction chemotherapy for ALL
Congenital Thrombophilia
Conflicting data. Routine thrombophilia screening is not recommended outside of a trial setting
Reducing risk of VTE
Simple
Early mobilization
Good hydration
Prompt removal of central lines at completion of treatment
Adolescents: Consider compression stockings
Discontinue COCP at diagnosis and use alternative
Lines
Internal port preferred to tunneled line for children at high risk of VTE
Tunneled line preferred to PICC for children with cancer
Avoid femoral access
No evidence for waiting until end of ALL induction chemo before line insertion
Antithrombin Replacement
FFP is not recommended for asparaginase
Insufficient evidence to support AT concentrate for asparaginase
Therefore, do not check AT levels
Routine thromboprophylaxis
Not recommended in children. Consider in adolescents at high risk.
Management of VTE
Central line-related VTE
Removal of line is not necessary if it still required, in a good position and functioning well
Symptomatic clot should be treated with 3 month’s anticoagulation
Insufficient evidence to recommend subsequent prophylactic doses if line remains in
Cerebral Venous Thrombosis (CVT)
Standard anticoagulation recommended, minimum of 3 months
AC is not contraindicated in presence of ICH unless risk of further bleeding > benefit.
VTE at other sites
Initial 3 months treatment
Consider treatment beyond three months if ongoing active cancer or other risks
Incidental VTE finding
Treat as for symptomatic VTE
If solely line-related, consider monitoring initially to see if AC required.
Choice of anticoagulant
LMWH treatment of choice
Routine measurement of Anti-Xa levels (0.5-1.0) recommended for children
Trials of DOACs underway.
Antithrombin Replacement
Routine AT replacement is not recommended during LMWH/UFH treatment
AC around time of invasive procedures
Stopping of LMWH / warfarin same as for adults
AC and thrombocytopenia
Continue whilst plt count >50
Use platelet transfusion to support >50 if life-threatening VTE within last 1-3 months.
Consider 50% dose when plt count 25-50
Re-exposure to asparaginase following VTE
Further doses may be given but should be covered by prophylactic or Rx-dose LMWH
This AC should be continued for 3 weeks following a dose of peg-asparaginase
Thrombocytopenia and coagulopathies
Plt thresholds prior to LP
Follow other BCSH guidelines
Monitoring for coagulopathies
FBC, Film, PT, APTT, FGN should be performed on all children with new malignancy
FVIII and VWF should be performed on all children with suspected Wilms tumour
Evidence of DIC should be sought in acute leukaemia
Repeat testing after starting treatment only indicated in presence of abnormal bleeding
In absence of the above, no need to recheck clotting prior to surgery or LP in ALL patients
Fibrinogen supplementation
Replace if <1g/l and due surgery or at high risk of bleeding
Choice of menstrual suppression
Progestogens should be considered first – medroxyprogesterone or norethisterone
Insufficient evidence to recommend routine use of gonadotropin-releasing hormone analogues over progesterone for purely fertility preservation purposes.