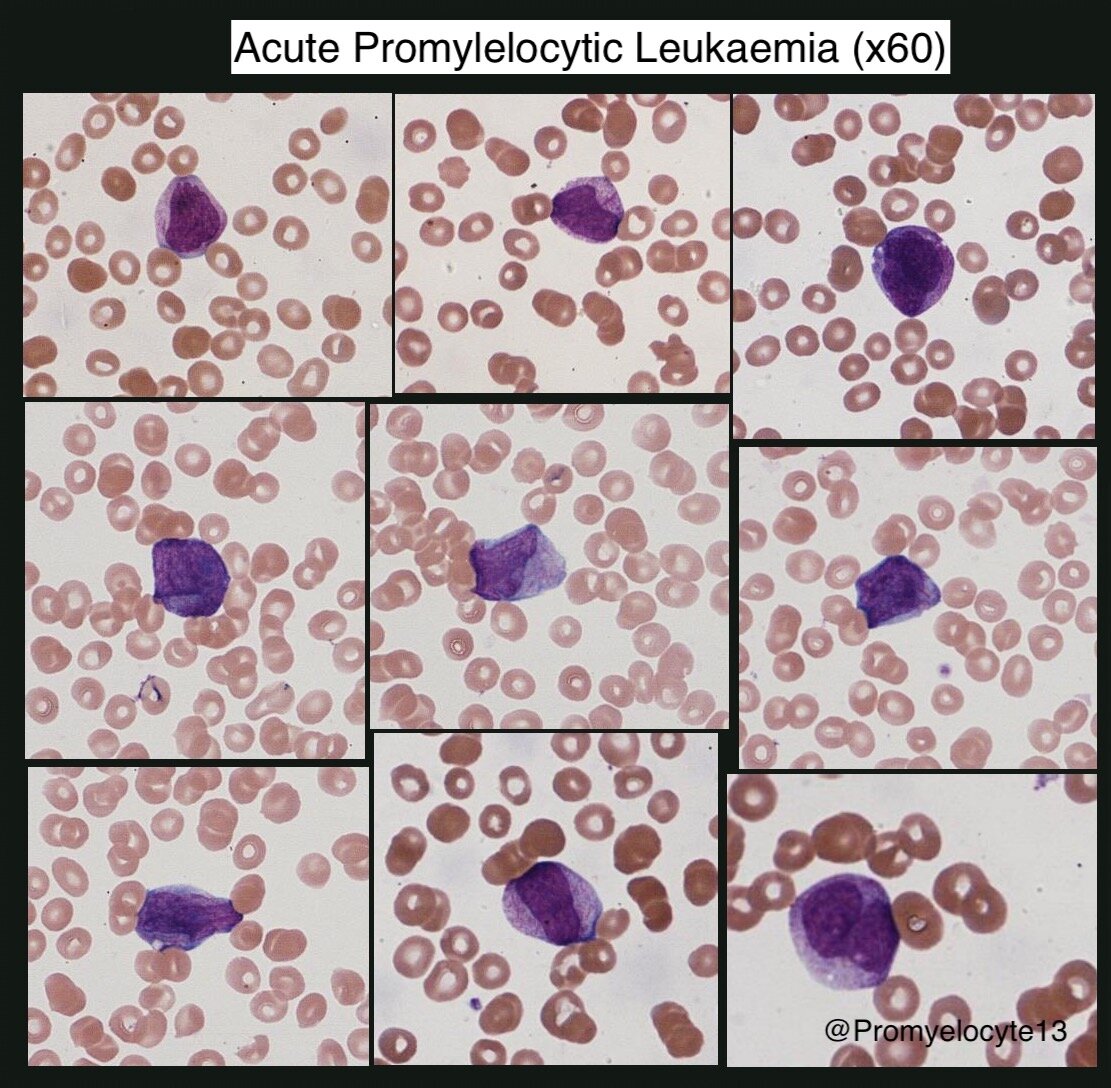
Acute Promyelocytic Leukaemia (APML) (Blood 2009)
CD45+, MPO+, CD117+, CD33+, Aberrant CD9+
CD34-, HLADR-
Intro
10-15% of AML cases
Usually young, with leukopenia and life-threatening coagulopathy
A balanced translocation between PML (Promyelocyte) & RARA (Retinoic Acid Receptor-a) generates a fusion protein —> leukaemic promyelocytes have the unique ability to differentiate when exposed to retinoic acid.
Diagnostic Investigations
Morphology
Kidney-shaped/bi-lobed nucleus, cytoplasm densely packed with granules/auer rods
Hypogranular variant exists, differential diagnosis of acute monocytic leukaemia
Immunophenotype
CD45+, MPO+, CD117+, CD33+, CD13+, CD64+/-, Aberrant CD9+
CD34-, HLADR-, CD11b-, CD11c-
Cytogenetics
t(15;17)
PCR as gold standard – able to detect PML-RARA in leukopenic patients. Takes 48 hours.
FISH – can give an answer in 6 hours
Karyotyping – expensive and time consuming
Risk Classification
WBC <10 – Low to intermediate risk disease
WBC >10 – High risk disease
Supportive Treatment
Coagulopathic bleeding responsible for 50-60% of early deaths (CNS, lung and GI)
Risk of bleeding may persist up to 20 days
Twice or thrice daily FBC, PT, APTT and FGN
Keep platelets >30-50
Keep PT/APTT normal
Keep FGN >1.5g/l
Definitive Treatment
Treatment should start based on morphological assessment, do not wait for FISH
1st line options
ATRA + Idarubicin (AIDA)
ATRA + Arsenic (ATO) - NICE approved 1st line for low risk disease only (as of June 2018)
ATRA
Overrides the t(15;17) protein induced blockade of the retinoic acid receptor
45mg/m2 daily in two divided doses to start on day 1
Continued until haematological CR and for a maximum of 60 days
Idarubicin
12mg/m2 on days 2,4,6 and 8. Or start on day 1 if WBC >10
Arsenic
Arsenic degrades the fusion protein and induces apoptosis
ATRA + Arsenic is a highly effective (97% CR) non-chemo regimen
ATRA Toxicity
Pseudotumour Cerebri
Usually patients <20 y.o.
Severe headache, nausea, vomiting and visual disturbance
Hepatoxicity
Bili/ALT/AlkP >5x the ULN
ATRA/APL Differentiation Syndrome
10 days after starting ATRA
Fluid retention, capillary leak – cough, hypoxia, effusion, oedema, weight gain, fever
Associated with rising WBC count. Risk lower when ATRA given with chemo.
Rx: Stop ATRA, give IV Dexamethasone, Cautious re-introduction when Sx resolve
Other adverse effects
Rash (Sweet’s Syn)
Pancreatitis, hypercalcaemia, bone marrow necrosis
Consolidation & F/up
>90% CR after induction + 2 cycles of consolidation.
No role for transplant in CR1
MRD monitoring 3-monthly for two years after completion of treatment
Relapse inevitable if PCR positive in two consecutive samples —> treat at molecular relapse.
At relapse
Induce 2nd CR with aim to harvest PCR negative cells for autograft
Consider Allograft in patients in whom PCR negativity cannot be achieved.