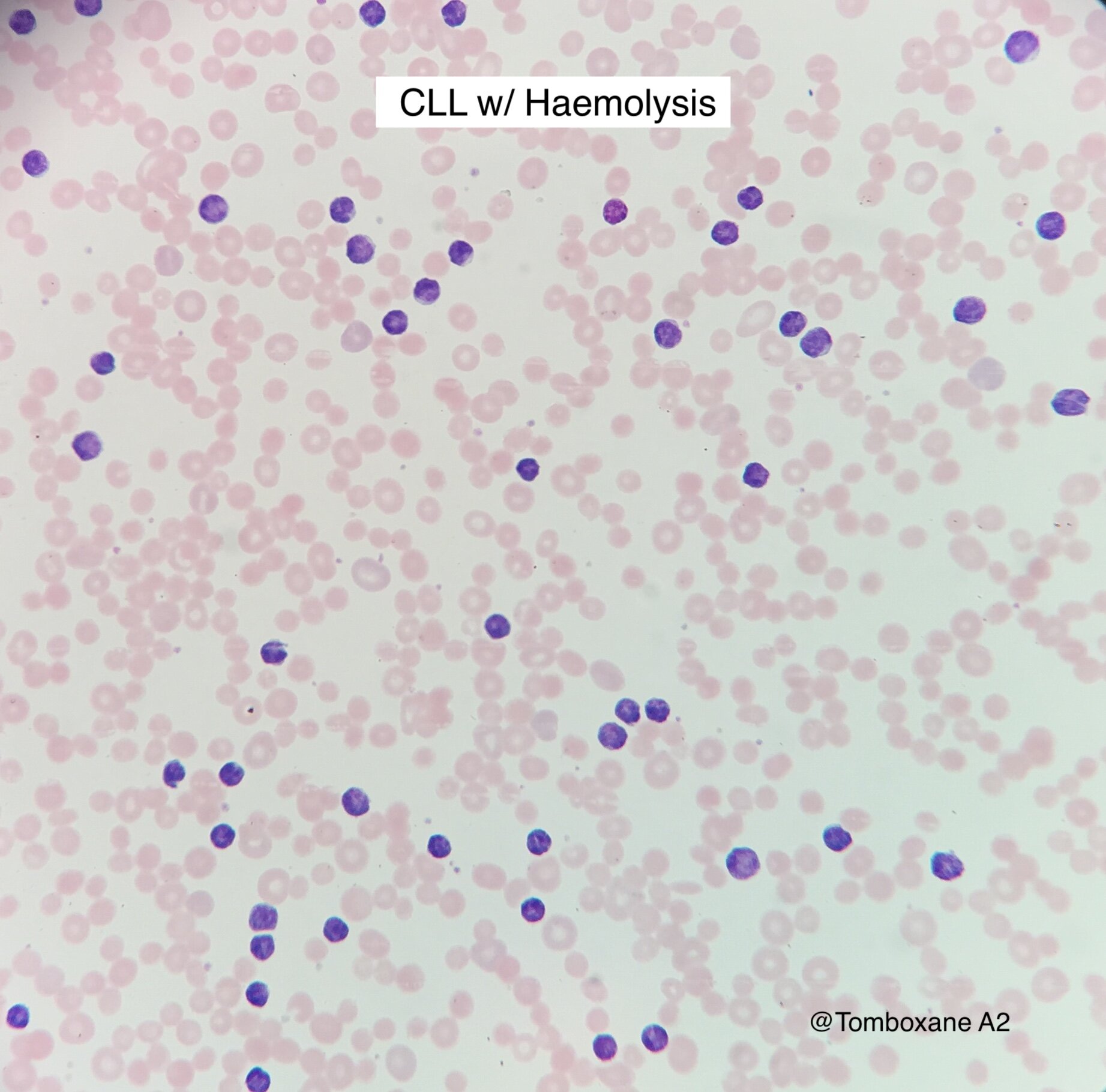
Intro
Incidence 4.2 per 100,000 per year in UK
Median age of presentation 72
Men > Women
Caucasian > other ethnic groups
>80% incidental diagnosis
Diagnosis
>5 x10e9/l circulating clonal B cells persisting for >3 months and of characteristic immunophenotype.
CLL Score (now outdated for many centres, as different CD markers often used)
Surface Ig weak, CD5+, CD23+, CD79b-, FMC7-
One point for each
92% of CLL scores 4 or 5
Prolymphocytes:
>15% prolymphocytes = Prolymphocytic progression of CLL (as of 2022, this replaces B-PLL)
DDx for CD5+ LPD with a low score = atypical CLL, mantle cell, marginal zone
cMBL = clinical CD5+ monoclonal B cell lymphocytosis
MBL detectable on a routine FBC but <5 x109/l monoclonal cells
WHO 2016 splits <0.5x109/l (no concern) from 0.5-5 x109/l (annual FBC monitoring)
Staging
BINET
A: <3 lymphoid areas
B: 3 or more lymphoid areas
C: Hb <100 or Plt <100
(Five lymphoid areas are: uni or bilat. cervical, axillary, inguinal, hepatomeg, splenomeg)
RAI
0: Low risk: Lymphocytosis only
I: Intermediate: Lymphadenopathy
II: Intermediate: Hepatomeg or splenomeg + lymphocytosis
III/IV: High risk: Hb <110 or Plt <100
Investigations
Asymptomatic Stage A patients
FBC, reticulocyte, DAT, biochemistry, immunoglobulins, Hep B/C, HIV
Flow cytometry
TP53 deletion (FISH)
Lymph node biopsy only if diagnostic uncertainty
CT not required at diagnosis (perform later pre-treatment)
Diagnosing transformation
5-15% of CLL transforms to DLBCL-like or HL-like
May be localized, biopsy the suspicious site
PET-CT may help identify best biopsy site.
Assessing prognosis
Binet / Rai systems predict outcome in advanced stage patients, but poor for early stage
Other factors:
Age, Gender, PS, co-morbidities
Marrow failure, immunodeficiency, autoimmunity, biomarkers
Type of treatment, Response, Toxicity, MRD status
Prognostic data should not influence timing of first treatment
Biomarkers include IGHV gene analysis, B2M, CD38 positivity
TP53
TP53 loss (del 17p13.1) occurs in 5-10% of patients at diagnosis, 30% in fludarabine refractory patients
TP53 mutation occurs in an additional 5% at diagnosis, 12% of refractory patients
Both TP53 loss and mutation indicate high risk disease, associated with lower response rates and short PFS when treated with chemoimmunotherapy (e.g. FCR or R-Benda).
IGHV
IGHV gene mutations confer a better prognosis
?functional cure in 50% treated with FCR, and excellent remissions with O-Venetoclax
‘IGHV unmutated’ is associated with a poorer prognosis.
Management
Indications to treat:
Lymphocyte doubling time <6 months (provided starting Lymph count >30)
Lymphocyte count increased >50% in 2 months
Progressive marrow failure
Massive (>6cm below costal margin) splenomegaly
Massive (>10cm) lymph nodes
Autoimmune anaemia / thrombocytopenia not controlled by standard therapy
Constitutional symptoms
Wt loss >10% in 6 months, fatigue (PS 2 or worse), Fever >38 for 2 wks, night sweats for >1 month.
(NOT indications to treat - High lymphocyte count (in absence of rapid doubling time), hyperviscosity and hypogammaglobulinaemia (in absence of recurrent infection))
Choosing therapy
No single choice, patient-by-patient
Age, co-morbidities, creatinine clearance, PS, susceptibility to infection
Important to achieve maximal response
Achieving MRD negative remission is an independent marker for OS and PFS
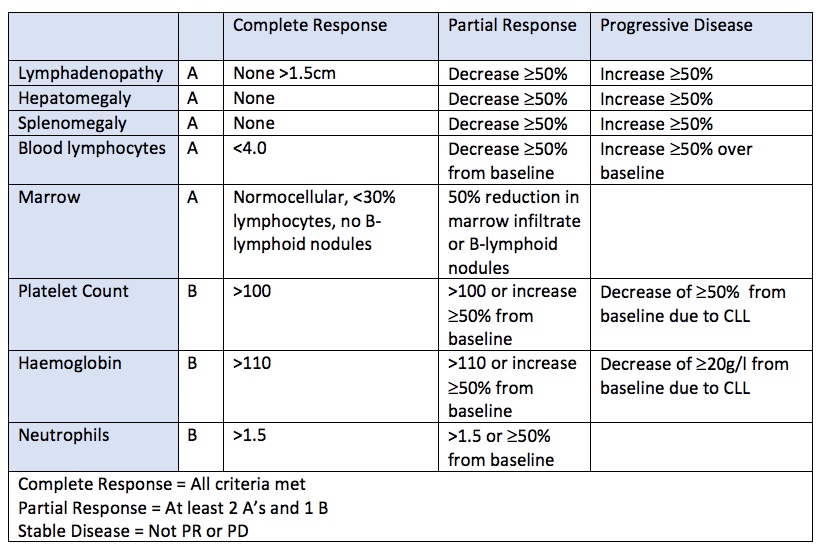
Defining response
IWCLL criteria for CR, PR and progressive disease
Relapse = disease progression at least 6 months after achieving CR / PR
Refractory = treatment failure or disease progression less than 6 months after CR/PR
Treatment not required for Early / Stage A Disease
6 monthly review in first year to assess rate of progress
Then annually if no concern, GP or hospital
Address patient and family concerns around leukaemia diagnosis and implications
Treatment Principles
Irradiated blood products
For life: purine analogies, bendamustine, campath
3 months post-conditioning for autograft
MDT approach
Offer clinical trial
Front-line Treament Options (Subject to frequent change!)
Targeted therapies +/- Immunotherapy have replaced traditional cytotoxic chemotherapy and have softened the distinctions between fit / unfit and TP53/noTP53. However some NICE approvals are still based on these groups.
UK Patients without TP53 abnormality
Ibrutinib + Venetoclax, fixed duration (15 months) (NICE 2023, CAPTIVATE 2022, GLOW 2022)
Zanubrutinib if ‘FCR or BR unsuitable’ (NICE 2023, SEQUOIA 2022)
Acalabrutinib if ‘FCR or BR unsuitable’ (NICE 2021, ELEVATE-TN)
Obinutuzumab-Venetoclax if ‘FCR or BR unsuitable’ (NICE 2020, CLL13, CLL14 + f/up 1 yr later)
(Watch this space - CLL13 (FCR v BR v O-Ven v R-Ven v O-Ibrut-Ven in fit pts w/out TP53))
UK Patients with 17p / TP53 disruption (deletions and/or mutations)
Ibrutinib + Venetoclax, fixed duration (15 months) (NICE 2023, CAPTIVATE 2022, GLOW 2022)
Zanubrutinib (NICE 2023, SEQUOIA 2022)
Acalabrutinib (NICE 2021)
Obinutuzumab-Venetoclax (1yr) (NICE 2020, CLL13, CLL14 + f/up 1 yr later)
Ibrutinib (NICE 2017)
Other approved options, less commonly used first line in UK now:
R-Idelalisib - acceptable alternative if specific issues prevent others (e.g. cardiac disease, warfarin use)
Obinutuzumab-Chlorambucil (CLL11 - PFS and TTNT benefit vs R-Chlormabucil)
FCR (CLL10)
R-Bendamustine
Maintenance in CLL?
No. Anti-CD20 antibodies and lenalidomide have been trialed - increase PFS but no effect on OS seen, and need to weigh the long-term toxicity
Relapse/Refractory Options (Subject to frequent change!)
Options (Guideline has recommendations on sequencing):
Zanubrutinib (NICE 2023, ALPINE 2023)
Acalabrutinib (ASCEND, ELEVATE-RR, NICE 2021)
Rituximab-Venetoclax (2yrs) (MURANO 2018, NICE 2019)
Ibrutinib
R-Idelasilib
When to consider allogeneic stem cell transplant?
Fit patients who have failed at least 2 of :chemoimmunotherapy, BTKi, BCL2i
Fit patients with Richter transformation
Supportive Care
Anti-microbial prophylaxis
PCP prophylaxis for at least first 12 months of BTKi
Aciclovir prophylaxis with intensive treatment or low CD4 counts
Azoles avoided in combination with BTKi and BCL2i due to interactions
Hypogammagloublinaemia
Prophylactic antibiotics for recurrent infections
Consider immunoglobulin replacement 3-4 weekly if low IgG (<4g/l) and recurrent infection. Discontinue after 1 year if not reduction in rates of infection.
Vaccination
Annual flu vaccination
Pneumococcal vaccination - Prevnar13 followed by Pneumovax23 two months later
Shingles - non-live vaccine now available (Shingrix)
Drugs: A few notes
Covalent Bruton Tyrosine Kinase inhibitors (BTKi) (Also see here)
Ibrutinib, Alcalabrutinib, Zanubrutinib
SE: acquired platelet function defect, hold for 5-7 days before interventional procedures / surgery
SE: Petechial rash, easy bruising & arthralgia usually improve with time
SE: Hypertension, AF & VT (including sudden cardiac death)
SE: With alcalabrutinib also look for headache, gastritis. But probabaly less cardiac side effects than ibrutinib (N.B. new tablet formulation of acala no longer has an issue with concurrent use of PPI’s)
Avoid CYP3A4 inhibitors - warfarin, fish oils, Vitamin E, St Johns Wort
Grapefruit and Seville oranges must be avoided
Non-Covalent BTKi
e.g. Pirtobrutinib
Alternative, reversible mechanism of binding BTK compared to covalent inhibitors (review paper 2021)
—> Can still be effective after resistance has developed to covalent BTKi’s
Typical BTKi side effects (bruising, rash, arthralgai, AF) may be less common and less severe.
BCL2 inhibtior - Venetoclax
MOA: Inhibitor of BCL2. BCL2 is a anti-apoptotic protein that is overexpressed in cells of many haematological malignancies. Apoptosis is reduced in these cells because BCL2 sequesters the pro-apoptotic protein BAX. —> Inihibiting BCL2 releases BAX which leads to cell death.
Significant risk of tumour lysis (—> dose escalated slowly over course of first cycle)
Ibrutinib + Venetoclax
Proposed to be synergistic when given in combination
Ibrutinib drives CLL out of lymph nodes, into peripheral blood where more sensitive to venetoclax
BTK inhibition makes CLL cells more dependent on BCL2 —> increased sensitivity to venetoclax
A 3 month lead in with ibrutinib reduces the risk of tumour lysis syndrome associated with venetoclax
PI3 Kinase Inhibitors - Idelasilib
May trigger life-threatening pneumonitis or colitis
Monitor liver function
Monitor CMV PCR’s
Use PCP prophylaxis
A Few Trial notes
Phase 3, First line CLL for fit patients without TP53 disruption
926 patiens. 1:1:1:1 randomisation:
FCRx6 or R-Benda x6
R-Venetoclax x12
O-Venetoclax x12
Venetolax-O-Ibrutinib x12
At 15 months, uMRD V-O-I (92%) & O-V (86%) vs R-V (57%) & Chemo (53%)
3-yr PFS was V-O-I (90.5%) & O-V (87.7%) vs (80%) & Chemo (75.5%)
Grade 3/4 infection was V-O-I (21%) vs O-V (13%)
Phase 3, R/R CLL, Ibrutinib vs Zanubritinib
2yr PFS: 78% zanubrutinib, 65% ibrutinib
Rates of drug discontinuation + cardiac events lower with zanubrutinib vs ibrutinib
Phase 3, First line CLL. Ibrutinib+Venetoclax vs O-Chlormabucil
211 patient, >65 yo (or high frailty score (CIRS) or CrCl <70ml/min)
Excluded del17p and TP53 mutation patients.
undetectable MRD (uMRD) I+V 55% in BM
2-yr I+V OS 90%, PFS 84%
Phase 3, First line CLL. Zanubrutinib vs R-Benda except all 17p del got Zanu (arm C)
590 patients, >65yo or <65 with co-morbidity
At 2 yrs, median PFS not reached for either arm but superior for zanubrutinib
50% neutropenia with zanubrutinib
Phase 2, First line CLL. 15 months Ibrutinib + 12 months Venetoclax
160 patients, <70 yo only in trial. 92% completed full treatment.
CR 55%. For those in CR, undetectable MRD (uMRD) was 90% in PB and 72% in BM.
2-yr OS 98%, PFS 95%
Phase 3, First Line CLL. O-Acala vs Acala va O-Chlorambucil
535 patients, >65 yo
2yr PFS 93% O-A, 87% A, 47% O-C
5 yr f/up: Median OS not yet reached for any arm. Est. 60 month OS 90% A-O, 84% A, 82% O-C
Phase 2, First line CLL. Up to 24 cycles of ibrutinib + venetoclax
80 high risk patients (del17p, TP53 mutation, del11q, unmutated IGHV or Age >65)
CR 88%. Undetectable MRD (uMRD) was 61% in BM
Only 3 patients completed 24 cycles of treatment.
CLL14 2019
12 months Venetoclax + 6x Obinutuzumab vs. 12 months Chlorambucil + 6x Obinutuzumab
432 patients
O-Venetoclax showed higher rates of complete response, MRD negativity and longer PFS
Follow-up off treatment confirmed longer PFS (median PFS not reached in O-V, 35 months for O-C)
Phase 3, R/R CLL. R-Venetoclax vs R-Benda
389 patients
2-yr PFS 84.9% R-Ven vs 36.3% R-Benda
COMPLEMENT-1 2015
Chlorambucil-Ofatunumab vs Chlormabucil alone
Combo better PFS but no difference in OS
Hard to compare to CLL11 as different dose of chlorambucil used
CLL11 2014
Chlorambucil-Obinutuzumab vs Chlorambucil-Rituximab vs Chlormabucil alone
Chlorambucil-Obinutuzumab had superior PFS and TTNT
Infusion reactions more common with obinutuzumab
CLL10 2013
FCR x6 vs R-Benda x6
FCR superior for ORR, MRD-neg remissions, length of first remission in young, fit patients
FCR arm had more serious adverse events
Overall survival similar for both arms