if you would like to support my site hosting fees and help keep Haembase #FOAMed forever you can do so here!
AML (BSH 2015, ELN 2022, BSH 2022, BSH 2022(2))
Precursors – CD34+, CD38+, CD117+, CD133+, HLA-DR+
Granulocytes – CD13+, CD15+, CD16+, CD33+, CD65+, cMPO+
Monocytes – NSE+, CD11c+, CD14+, CD64+, lysozyme, CD4+, CD11b+, CD36+
Megakaryocytes – CD41+, CD61+, CD42+
Erythroid – CD235a+
Intro
Median Age: 70 years
Age <65: 3-8 cases per 100,000 adults per year. 40% 5-year OS
Age >65: 9-17 cases per 100,000 per year. 10% 5-year OS
WHO-HAEM5 Classification
(Note: ICC classification also published the same year, see below)
Notes
>20% blasts no longer required for AML with defining genetic abnormalities (except CEBPA, BCR-ABL1)
‘post cytotoxic therapy’ (pCT) can be added as suffix to myeloid diagnoses where indicated by medical history, e.g. CMML-pCT.
See WHO-HAEM5 for detailed sub-sections:
AML, myelodysplasia-related: list of defining abnormalities.
AML, definitions by differentiation
Myeloid neoplasms associated with germline predispositions
Mixed lineage phenotypes
ICC 2022 Classification
Unfortunately two classification systems were published in WHO and ICC
They are the same in principle, e.g. genetics > morphology, and the change to blast % cutoffs
The ELN have opted to use the ICC. You can see the ICC 2022 Classification here.
Clonal Haemtopoeisis of Indeterminate Potential (CHIP)
Found from large, population-level cohorts of elderly, seemingly healthy subjects
May behave like MGUS / MBL in terms of risk to progression of AML
Commonly DNMT3A, ASXL1, TET2, SF3B1, SRSF2
Diagnosis
Basics
FBC, film
Biochemistry, Coag,
HIV, Hep A/B/C
HLA-typing
Urine dip, Pregnancy test
CXR
Oocyte / Sperm cryopreservation
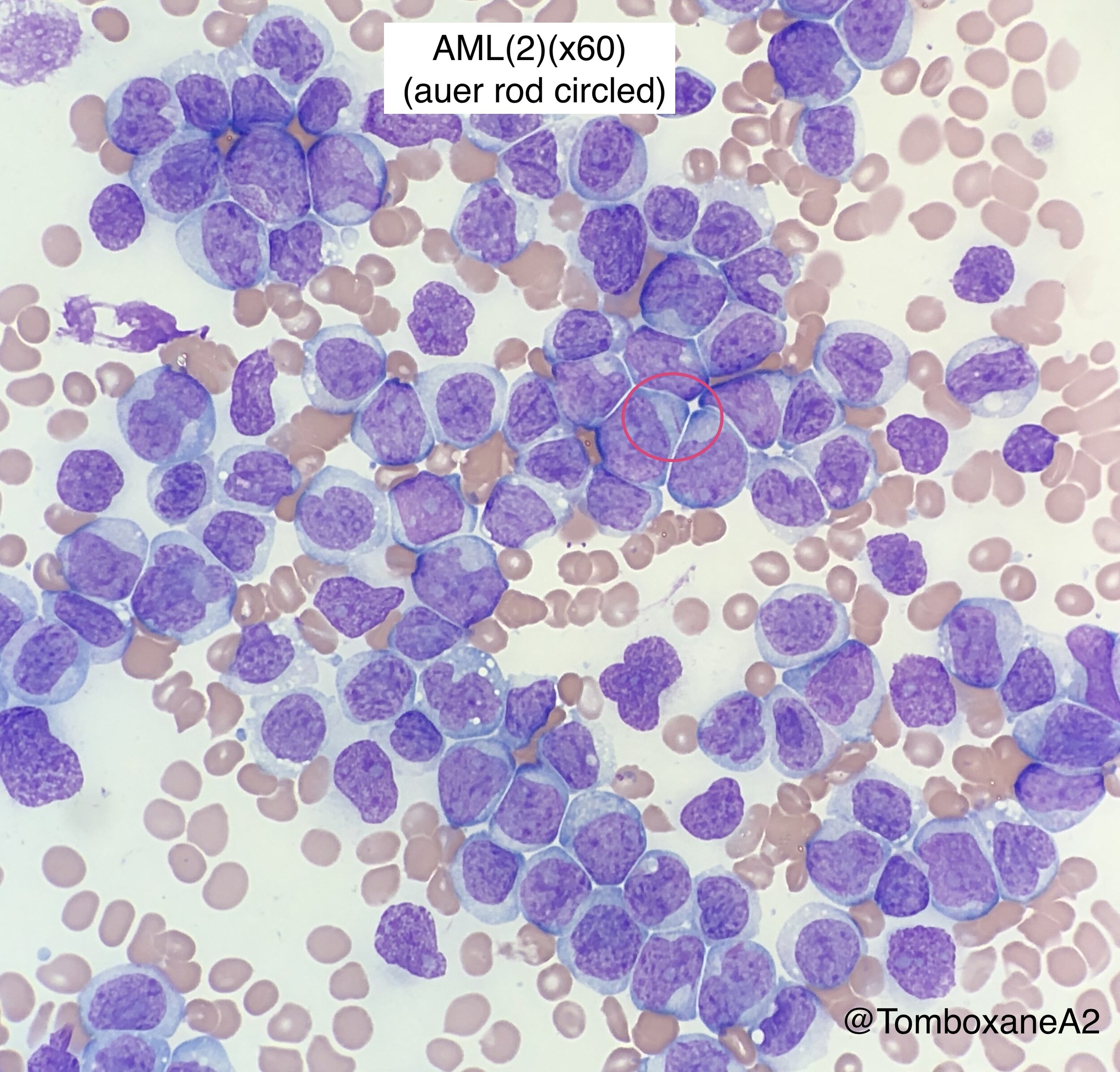
Morphology
Aspirate mandatory, trephine optional
May-Grunwald-Giemsa or Wright-Giemsa stain
>20% Blasts in marrow for morphological AML diagnosis (Exceptions: t(15;17), t(8;21) and inv(16))
Immunophenotyping
Used to determine lineage
>20% of leukaemic cells expressing a marker counted as positive, as a general rule
Flow blast count is not a substitute for morphological count.
Examples of specific phenotypes:
Acute megakaryoblastic - CD41+, CD61+ (CD42 usually lost on megakaryoblasts)
t(8;21) RUNX1:RUNX1T1 - CD19+, CD56+/-, Strong CD34+, Weak CD33+, MPO+
t(15;17) APML - High SSC, CD33+, CD13+, CD117+, MPO+, CD34-, HLA-DR-, CD11b-
BPDCN - CD123+, CD4+, CD56+, HLA-DR+, CD34-, CD13-
Cytogenetics
55% of AML cases have detectable chromosome abnormalities
Minimum of 20 metaphases must be examined for a normal karyotype
Molecular Cytogenetics (FISH)
PML-RARA, RUNX1-RUNX1T1, CBFB-MYH11
KMT2A fusion gene, 5q deletion, 7q deletion
Molecular Genetics (RT-PCR)
Detects fusion genes, such as those listed under FISH
Detects somatic mutations – NPM1, FLT3, CEBPA, KMT2A, RUNX1, KIT, TET2, IDH1
NPM1, FLT3 and CEBPA should be tested as a minimum in pts with normal cytogenetics
Can be use for MRD monitoring of AML cases with NPM1, PML::RARA or CBF / KMT2A fusions
Genome-wide studies
Research methods for identification of new genetic abnormalities
Single Nucleotide Polymorphism (SNP)-arrays
High-throughput DNA sequencing
Large scale RNA interference screens
Testing paired tumour + germline (e.g. skin) samples to investigate for germline predisposition, consider in AML cases with RUNX1, CEBPA, DDX41, ANKRD26, ETV6 or GATA2 mutations present.
Minimum genetic tests for all new AML patients in 2022 (BSH 2022)
FISH/PCR/Karyotype for Inv16 (CBFB::MYH11), t(8;21) (RUNX1::RUNX1T1) & KMT2A (MLL)
Karyotype
Molecular for FLT3-ITD, FLT3-TKD, NPM1
NGS Panel to include ASXL1, BCOR, EZH2, RUNX1, SF3B1, SRSF2, STAG2, U2AF1, ZRSR2, TP53, FLT3, IDH1, IDH2, DNMT3a and WT1
Some Significant Genetic Mutations
PML-RARA translocation
t(15;17) – APML
NPM1 mutation
1o genetic lesions (“Class II”) impairing haemopoietic differentiation
CEBPA mutation
1o genetic lesions (“Class II”) impairing haemopoietic differentiation
FLT3-ITD
“Class I” mutation found in approx. 1/3 of AML cases.
RUNX1
Alters transcription activity
IDH1&2
Mutations lead to arrest of haematopoietic differentiation
KMT2A (prev. MLL)
Lysine Methyl Transferase 2A.
5% of AML cases. 5yr OS 38% in AML17+19.
MRD testing becoming available (as of 2025)
DNMT3A, TET2, ASXL1
Often present in preleukaemic stem cells —> may persist after Rx
Germline predisposition (UKCGG 2023, ELN 2022)
Increasing number of gene mutations associated with heritable risk of leukaemia
Somatic gene varaints w/ variant allele frequency (VAF) approaching 50% suggest possible germline involvement
Potential cases will usually be reviewed at a specialist haematopathology MDT
These patients may warrant germline testing, e.g. skin biopsy
UKCGG: Offer germline testing for pathogenic or likely pathogenic variants with VAF >20-30%
Consenting for germline testing
Ideally start conversation prior to somatic testing, mandatory prior to germline testing itself
Source of germline samples
Skin biopsy or remission bone marrow aspirate are preferred
Blood and saliva unsuitable for testing in setting of haematological malignancies
Other samples currently have various limitations to testing in UK. Examples discussed in link above.
Examples
DDX41 variants, first reported 2015, are now the most strongly associated with germline predispositions
Present in >5% of adult AML. Median age of onset in later life.
Modest disease penetrance (40% by age of 90 and <30% patients report a FHx of haem malig)
Other potential genes are RUNX1, CEBPA, ETV6, GATA2, TP53, TERT, TERC, ANKRD26, CHEK2
Prognostic Factors
Patient-Related Factors (predict TRM)
Age
Co-morbidities
AML-Related Factors (predict response to treatment)
WBC
Prior MDS
Prior Cytotoxic chemotherapy
Cytogenetics
Strongest AML-related prognostic factor predicting response to initial therapy
Favourable, Intermediate and Adverse
Molecular Genetics
Becomes relevant when patient is cytogenetically normal (CN-AML)
E.g. FLT3-ITD poorer prognosis
E.g. NPM1 and CEBPA mutations have favourable prognosis
Minimal Residual Disease (MRD) monitoring
Flow 1 log less sensitive but more available than RT-PCR
MRD status prior to allograft significantly affects survival post-transplant
Personalised risk calculator available from the Sanger Institute (currently a reserach tool only)
ELN Risk Stratification by Genetics 2022
ELN 2022. Note allelic ratios removed from the FLT3 mutations (compared to 2017)
ELN Response Assessment
Performed between day 21-28 of induction chemotherapy (e.g. DA 3+7)
CR = marrow blasts <5%, Neut >1, Plt >100, No circulating blasts
CR with MRD negativity
CRh, CRi, PR, No Response (see ELN for details, pg 1361)
(N.B. NPM1 MRD positivity after 2 cycles of induction associated with very poor prognosis)
Management
Adults 18-60 years old
Induction therapy achieves CR in 60-80% of adults <60 y.o. (TRM 5-10%)
Common UK regimens include FLAG-Ida-GO and DA-GO
Addition of small molecule drugs for pts with relevant mutations (see note below)
Postremission therapy
Up to 3 further cycles treatment, e.g. 2nd DA followed by 2 x HD Cytarabine
Allograft in 1st CR offers significant OS benefit if intermediate or adverse AML
Allograft TRM 15-50%
Allograft LT survival for adverse AML in 1st CR is 30% (but chemo alone dismal)
Adults >60 years old (BSH 2022)
Remission induction chemotherapy provides better QOL and longer survival than supportive care alone so offering induction chemo should be considered
All patients
Assess presence of frailty
Comprehensive geriatic assessment (GA) can aid decision making / sometimes prognosis
GA includes comorbidity, cognition, mental health, functional status, frailty, nutrition, polypharmacy, social support, quality of life. BSH good practice paper includes several of these scores in appendices.
Prophylactics: Quinolones, Aciclovir, Azole anti-fungals, Flu/Covid vaccination
60-74 years old
Standard induction chemo —> CR 50%, TRM 10-20%, 2-yr OS 50%
RIC Allograft has been performed up to age of 74
Venetoclax + Azacitidine. NICE approved 2022 for patients not fit for intensive induction. Based on VIALE-A trial 2020 - 400 pts, median age 76, CR rate 36%, CR+CRi 66%. Long-term outcomes awaited. Venetoclax most effective in NPM1 mutated, FLT3-ITD negative AML but approved for all cytogentic groups.
Ivosidenib + Azacitidine. NICE approved 2024 for IDH1 R132 mutated AML in patients who cannot have standard intensive chemotherapy. Based on AGILE 2022 - 140 pts, Ivo+Aza vs placebo+Aza, median OS 24 months for Ivo+Aza. No head-to-head trials vs Ven+Aza as of 2024.
>75 or not fit for intensive chemo
Venetoclax + Azacitidine. NICE approved 2022 for patients not fit for intensive induction. Based on VIALE-A trial 2020 - 400 pts, median age 76 (oldest 91), CR rate 36%, CR+CRi 66%.
Ivosidenib + Azacitidine. NICE approved 2024 for IDH1 R132 mutated AML in patients who cannot have standard intensive chemotherapy. Based on AGILE 2022 - 140 pts, Ivo+Aza vs placebo+Aza, median OS 24 months for Ivo+Aza. No head-to-head trials vs Ven+Aza as of 2024.
Azacitidine monotherapy if blasts 20-30% in marrow - CR 10-30%, median OS 6-12 months
Hydroxycarbamide
Supportive care alone
Relapse
Re-assess molecular status —> to aid consideration of small molecules, clinical trial
Therapy-Related AML
Many pathways, poorly understood but two groups stand out
5-7 years post alkylating agents or irradiation —> 5q or 7q deletion AML
2-3 years post topoisomerase II drugs —> MLL or RUNX1 AML
Poor prognosis
Often excluded from trials so data lacking. Allograft highest chance of long term survival
Relapsed AML
Majority of patients with a CR will relapse within 3 years
1-year survival 70% for favourable AML, 16% for adverse
Special situations
Hyperleukocytosis (WBC >100) – hydroxycarbamide until WBC <10-20
CNS involvement - <5% of patients. 3 x per week IT cytarabine until no blasts
Myeloid sarcoma – normal AML induction +/- radiotherapy
Supportive Care
Fungal, viral and bacterial prophylaxis
Platelet, red cell transfusions
Notes on NICE Approved Agents
Antibody-Drug Conjugates - e.g. Gemtuzumab ozogamicin (GO, Myelotarg)
Mechanism of Action
Combination of Anti-CD33 + Calicheamicin, a cytoxic antibiotic.
CD33 highly expressed on AML blasts, and increasingly less so as myeloid cells differentiate
It is not expressed on CD34+ pluripotent stem cells
On non-haemopoitic cells, CD33 is found on hepatocytes —> risk of VOD
GO - Approved for prev. untreated CD33+ AML, where patient is known to have favourable, intermediate or unknown cytogenetics at the start of treatment, or the results are not yet available. NICE 2018
Lipsomal Drug Preparations - e.g.CPX-351
Liposomal daunorubicin + cytarabine combination.
Thought better marrow take up and longer half-life (longer cytopenias as a result)
Approved for Therapy-related AML and AML with MDS-related change. NICE 2018
FLT3 Tyrosine Kinase Inhibitors – e.g. Midostaurin, Quizartinib, Gilteritinib
FLT3 mutations present in a third of AML cases
Midostaurin combinations
Midostaurin + DA approved for FLT3-ITD postive patients. RATIFY 2017. NICE 2018.
OPTIMISE-FLT3 opening in 2025: M-DA vs M-DA-GO vs M-FLAGida-GO
(Negative trial for midostaurin + Ven-LD Cytarabine. FLT3 subgroup may benefit. ALLG AMLM25)
Gilteritinib monotherapy
Approved for relapse. SE: 3-4% differention syn. ADMIRAL 2019. NICE 2020
Quizartinib + DA
Approved for newly diagnosed FLT3-ITD positive patients, followed by quizartinib monotherapy for maintenance. NICE 2024
Other FLT3 inhibitors not currently approved include Crenolanib, Sorafenib
IDH1 Inhibitors - e.g. Ivosidenib
Ivosidenib (IDH1 inhibitor)
IDH1 or IDH2 (Isocitrate DeHydrogenase) mutations present in 20% of AML cases. IDH is an enzyme in the kreb cycle. Mutant IDH1 and IDH2 produces an abnormal metabolite which blocks normal cell differentiation.
Ivosidenib + Azacitidine - Approved for patients not fit for standard induction regimens. NICE 2024
AML in Pregnancy
General Points
MDT approach
Diagnose as per the WHO classification
Treat without delay, DA(60) 3+10
Use actual body weight
Avoid quinolones, tetracyclines, sulphonamides
CMV negative products
Diagnosis in first trimester
Successful pregnancy outcome is unlikely and spontaneous pregnancy loss dangerous for patient (bleeding in thrombocytopenia / coagulopathy / infection)
Counsel patient on termination of pregnancy
Diagnosis at 12-24 weeks
Balance risks of foetal chemotherapy exposure against premature delivery
Chemotherapy in 2-3rd trimester rarely causes congenital malformation but does increase risk of late miscarriage, prematurity, fetal growth restriction and neonatal sepsis.
Where possible, deliver baby at least 3 weeks post-chemotherapy to reduce neonatal myelosuppression
Diagnosis beyond 32 weeks
Consider delivering baby first
NVD preferred over C-section
Active management of third stage of labour is recommended
Supportive therapies
Anti-emetics – Cyclizine preferred
Abx - Penicillin, cephalosporins, metronidazole, erythromycin safe in pregnancy
Anti-fungal – Ambisome preferred
emerging therapies / non-nice approved
(Notes from BJH 2018 and more recent ASH updates)
Key Principle
Only 3% of AML cases now have no detectable causative mutation —> Wide range of targeted therapies in development
Menin Inhibitors - eg Revumenib, Ziftomenib
Target KMT2A re-arrangements and NPM1 mutations
Disrupt the menin-KMT2A complex —> differentiation and apoptosis of AML cells (Hematol Rep 2024)
IDH2 Inhibitors
Enasidenib (IDH2 inhib) trialled in R/R AML —> Median OS 9 months (19 months for patients in CR)
Immune Checkpoint Inhibition – Nivolumab, Pembrolizumab
Nivolumab combined with azacitidine in older patients appears tolerable and some benefit.
CDK9 Inhibitors - Alvocidib
CDK9 regulates MCL1 expression. MCL1 is an anti-apoptotic protein.
E-selectin inhibitors - Uproleselan, others in development
Uproleselan - Ph3 trial, 380 R/R patients in combo with intensive chemo. No OS benefit. Possible benefit in primary refractory patients? ASH 2024
Hypomethylating Agents (other than azacitidine) - e.g. Decitabine
Ventoclax-Decitabine vs SOC for 1st line AML <60yo. Adverse risk group has higher 1st CR rate (ASH 2024)
Others
Monoclonals - e.g. Cusatuzumab (Anti-CD70) - outcomes awaited
Bispecific Antibodies – Flotezumab (CD123+CD3) - outcomes awaited
Smoothened inhibitors - Glasdegib - negative phase 3 trial (BRIGHT AML 1019, 2023)
HDAC inhibitors - Pracinostat - negative phase 3 trial (PRIMULA 2024)
CAR-T therapy – CD123 (IL3 receptor) present on 90% of blast cells - outcomes awaited
trial notes
MyeChild Trial (ASH 2024)
Paediatric AML. Phase 3. Added Gemtuzumab ozogamcin (GO) to mitoxantrone-cytarabine induction
CR 94% and 2-yr OS 88%
AML-19 (multiple publications, see below)
Phase 3. Patients <60yo or ‘fit’. First Line AML therapy. FLAG-Ida-GO vs DA-GO.
4 Questions:
Is the use of 2 doses of Myelotarg superior to 1 dose when combined with DA or FLAG-Ida?
No difference in CR rate or OS (Blood 2022)
Does FLAG-Ida+GO induction improve survival compared to DA(60)+GO?
FLAG-Ida+GO reduced relapse rate but no difference in OS. Subgroup with NPM1 or FLT3 mutation had better 3-yr OS with FLAG-Ida+GO (JCO 2024)
Does the addition of 1 or 2 courses of high dose Ara-C consolidation to 2 courses of FLAG-Ida improve survival?
In high-risk patients, is CPX-351 superior to FLAG-Ida at induction?
No difference in OS or EFS. RFS was longer with CPX-351. Exploratory subgroup showed longer OS in patients with MDS-related mutations. (Blood Advances 2023)
Midostaurin + DA(3+7) induction for FLT3-ITD AML. Placebo controlled.
Greatest impact when as close to diagnosis as possible. Maintenance therapy not effective.
Median OS in younger adults 74 months with midostaurin, 25 months with placebo
Only additional side effect was an increased rate of grade 3 rash/desquamation.