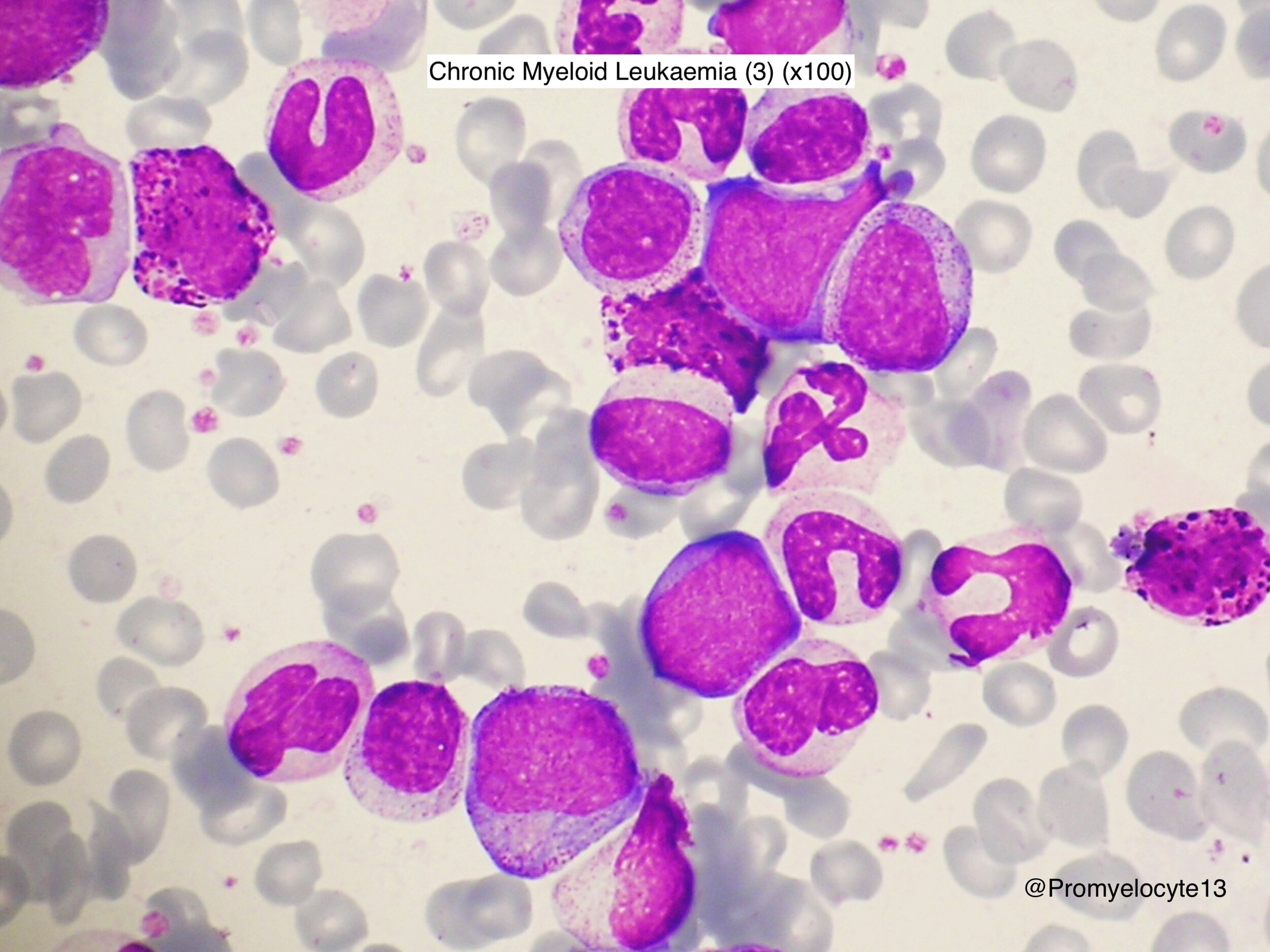
Pathophysiology
Part of ABL from 9 moves to the BCR on 22. (and part of 22 moves to 9)
Philadelphia (Ph) chromosome = the abnormal 22 that carries the BCR-ABL1 fusion gene
BCR-ABL1 fusion gene codes for a protein with excess tyrosine kinase activity
Ph chromosome is an acquired abnormality of a haemopoietic stem cell and so found in cells of both myeloid and lymphoid lineages.
Clinical Presentation
Median age at diagnosis = 57 years
20% of pts >70 yo, <5% of pts <18
50% diagnosed on incidental FBC finding
B symptoms, splenomegaly, symptoms of anaemia / thrombocytopenia
Terminology
Chronic Phase
Not accelerated or blastic phase
Accelerated Phase (ELN only, no longer exists in the WHO-HAEM5 classification)
Blasts 15-29% in blood or marrow
Basophils >20% in blood
Persistent thrombocytopenia <100 unrelated to therapy
Major route Additional chromosomal abnormalities (ACA) emerging in Ph+ cells while on treatment
Blastic Phase (ELN)
Blasts >30% in blood or marrow (or >20% in WHO-HAEM5)
Extramedullary blast proliferation, apart from spleen
Complete Haematologic Response (CHR)
Normal blood counts
Cytogenetic Response (CyR)
Assessed by chromosome banding analysis (CBA) of at least 20 marrow cell metaphases. Peripheral blood FISH is an acceptable alternative only when assessing Complete CyR (CCyR), defined as <1% BCR-ABL1 positive nuclei of at least 200 cells.
Molecular Response
Ratio of BCR-ABL1 transcripts to ABL1 transcripts, measured by quantitative reverse transcirptase PCR (RT-qPCR).
It is reported as BCR-ABL1 IS on a log scale, where 10%, 1%, 0.1%, 0.01%, 0.0032% and 0.001% correspond to a decrease of 1,2,3,4,4.5 & 5 logs below the standard baseline.
Major Molecular Response (MMR, MR3.0)
<0.1% BCR-ABL1 transcripts
Deep Molecular Response (DMR)
MR4.0 defined as:
either i) detectable disease with <0.01% BCR-ABL1 IS
or ii) undetectable disease in cDNA with >10,000 transcripts.
MR4.5 defined as:
either i) detectable disease with <0.0032% BCR-ABL1 IS
or ii) undetectable disease in cDNA with >32,000 transcripts.
MR5 defined as:
either i) detectable disease with <0.001% BCR-ABL1 IS
or ii) undetectable disease in cDNA with >100,000 transcripts.
Molecularly undetectable leukaemia
undetectable disease (must state number of transcripts analysed)
Treatment-Free Remission (TFR)
Emerging as the long-term goal of CML management for some patients
Diagnosis
initial work-up
Physical examination, Cardiovascular Disease risk assessment (e.g. Qrisk-3)
Bloods - inc Hep B & C screening, Cholesterol, lipid profile, HbA1c
ECG (TKI’s prolong QTc)
Bone MArrow biopsy
Aspirate
?Disease Phase - % Blasts, % Basophils
Trephine
Degree of fibrosis (prognostics)
Cytogenetics
To identify Philadelphia chromosome & Additional Chromosomal Abnormalities (ACA) in Ph+ cells
(‘Major Route’ ACA’s include extra Ph, trisomy 8, trisomy 19, isochromosome 17q)
Chromosome Band Analysis (CBA) and/or FISH
Molecular
Qualitative PCR to identify type of BCR-ABL1 transcripts (followed when assessing treatment response)
98% of patients have e13a2 and e14a2 transcripts
(terminology note: e13a2 = fusion between BCR exon 13 and ABL1 exon 2. Etc)
2-4% pts have atypical transcripts, e.g. e13a3 & e14a3 which lack ABL1 exon 2
(Further molecular testing can include BCR-ABL1 Kinase Domain (KD) mutation analysis. This is not indicated at diagnosis in chronic phase, but may be relevant at later date, e.g. suspected TKI resistance)
Note on TP53 and blast phase (all MPN’s, not just CML)
TP53 mutations are not uncommon in chronic phase of MPN’s
The high risk mutations seem to be the multi-hit and/or high copy number mutations
Review paper found multi-hit TP53 mutations in 0% of PV/ET, 37% MF, 81% Accel. phase and 90% of blast phase cases. (ASH 2024)
risk assessment
EUTOS Long Term Survival Score (ELTS) - score based on TKI-treated patients.
Designed to predict risk of dying from CML (leukaemia-related death)
Replaces Sokal, Hasford and EUTOS
High Risk = ELTS Score >2.2185
Cytogenetics may identify Additional Chromosomal Abnormalities in Ph+ Cells (ACA)
Major Route ACA = +8, +Ph, i(17q), +19, +17, +21
Minor Route ACA = -7/7q-, 11q23, 3q26.2
Presence of ACA in chronic phase is a warning of potentially more aggressive disease
Response to Treatment
Initial Monitoring
Fortnightly FBC until complete haematologic response (normal WBC), and to assess for haematological toxicity. Then every 3 months until MMR achieved, after which every 4-6 months in stable patients.
Response Milestones
See ELN 2025 guideline for detailed explanation of the nuance in intepreting milestones
Achieving MMR (<0.1%) predicts a CML-specific life expectancy the same as that of the general pop.
But Patients not meeting milestones do not necessarily need to switch treatment, needs careful consideration
E.g. this Leukaemia 2023 paper describe outcomes in patients not meeting the ELN milestones. Although outcomes are worse, the difference is smaller than anticipated and provides some reassurance for patients not able to switch TKI (eg. due to co-morbidities).
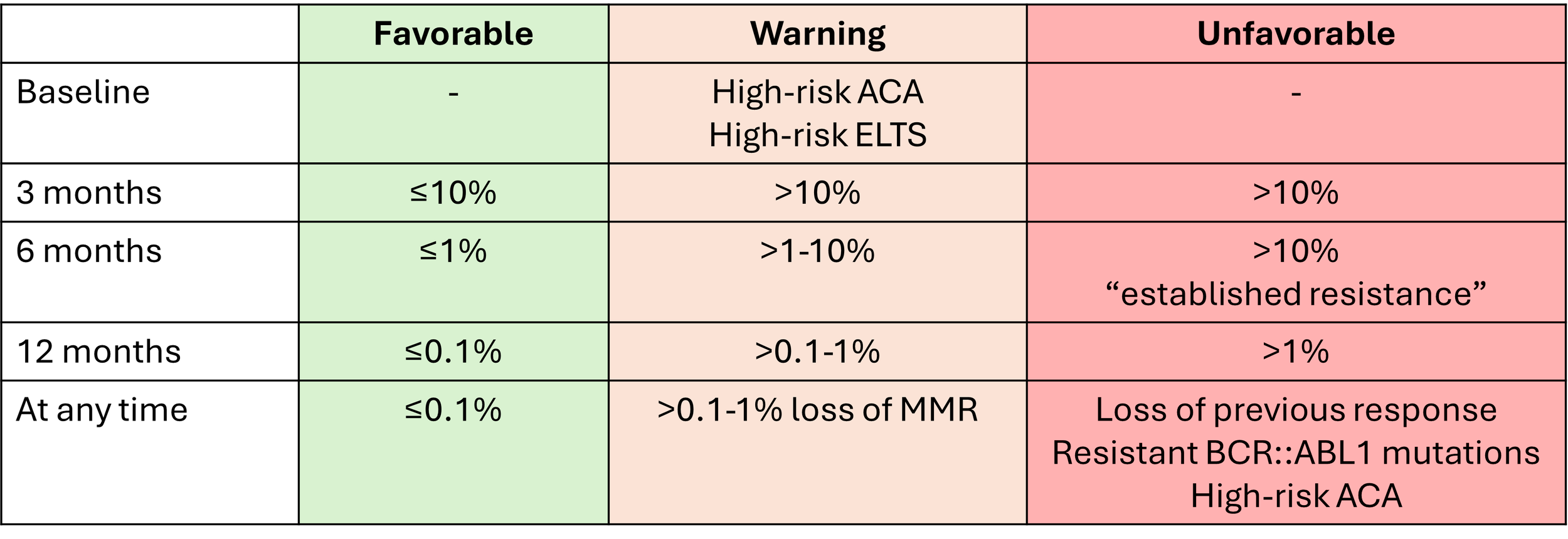
Table: Response milestones for 1st, 2nd and 3rd line TKI’s (ELN 2025)
Note minor change in terminology from previous ELN tables
Favorable - low risk of developing resistance: treatment switch not required
Warning - Possible risk of developing resistance: treatment switch may become necesary
Unfavorable - Higk risk of developing resistance: treatment switch preferred
Treatment Recommendations in diagnosis at chronic phase (outside of trial)
1st line
Any of following at standard dose:
Imatinib 400mg OD
Nilotinib 300mg BD
Dasatinib 100mg OD
HLA type patient and siblings if baseline warnings of high risk, major route CCA/Ph+
2nd line, intolerant to first
N.B Intolerance can often be managed with dose reduction of the 1st line TKI, consider this first if in MMR.
Alternative TKI, any of the following at standard or higher dose
Imatinib 400mg BD
Nilotinib 400mg BD
Dasatinib 100mg OD
Beyond 2nd line
Intolerance:
Try alternative 2nd gen TKI, introduced at a lower dose
Resistance:
Move on to asciminib or ponatinib
Test for TKI-resistance mutations
HLA type patient and siblings, unrelated donor search in preparation of potential allograft
At any time
If T315I mutation present use Ponatinib 45mg OD and consider allograft.
Others
Hydroxycarbamide can be used briefly whilst awaiting confirmation of diagnosis
Interferon: see below re: pregnancy
Cytotoxic chemotherapy never recommended in chronic phase
Busulfan not recommended
ELN guidance on Pregnancy & CML
Women who are pregnant
Individualised treatment
Stop TKI during first trimester as soon as pregnancy confirmed.
All TKI’s are teratogenic - hydrops fetalis known to occur with dasatinib
If patient in advanced disease, termination of pregnancy should be considered
IFN-a safe for use in pregnancy
Supportive care - involve obstetrics, thromboprophylaxis, leukopheresis
Breastfeeding
TKI’s contraindicated, low-level secretion in breast milk
Women who are planning pregnancy
Consider whether meet the criteria for treatment-free remission as per notes below
Substitution of TKI with IFN-a
Alternative methods for conception
Men who are planning fatherhood
No need to discontinue imatinib or 2nd gen TKI
No increased risk of congenital abnormalities in offspring
BSH guidance on Pregnancy & CML
Broad principles the same as ELN. Lots of useful additional considerations contained in the guideline, including a graphic on page 13 for planning pregnancy in women already diagnosed with CML.
Drug details & Side Effects
Patterns of side effects vary with different TKI’s. The BSH guideline contains lots of additional information, in particular take a look at table 2 (choice of TKI base on patient’s pre-existing co-morbidities) and table VII (TKI side effects and their management).
Three categories
Major grade 3/4 effects occurring in first phase of treatment and require temporary cessation of drug and dose reduction. About 10% patients unable to re-start.
Minor grade 1/2 effects beginning early in treatment. Chronic but tolerable although negatively affect quality of life.
Late ‘off-target’ effects on CVS, heart, lungs, liver, pancreas, immune defense, glucose and secondary malignancies
Imatinib (1st gen)
MOA: Competes with the ATP binding site of BCR-ABL1 kinase
Less potent than other TKI, but overall survival is no different
Off patent since Nov 2016 – 10x cheaper!
5-year Deep Molecular Response rate 35-70%
TIDAL2 Sudy – suggests switch to 2nd gen TKI if not <10% at three months
C/I: No absolute contraindications. Observe pts with renal or cardiac impairment closely
SE: Rash, oedema, diarrhoea, cramps, joint pain, fatigue. Probably safer than the others
Dasatinib (2nd gen)
More potent than imatinib, 4-year MMR-rate approx. 75% compared to 60% imatinib
Licensed dose is 100mg daily. Pleural effusions less common with 50mg and emerging evidence of similar efficacy.
C/I: Respiratory failure, Pleuro/pulmonary/pericardial disease
SE: 3-5% cardiac, 5% Pleural effusion per year, Pul HTN, opportunistic infections (neutrophil dysfunction), platelet function defect
Nilotinib (2nd gen)
More potent than imatinib, 4-year MMR-rate approx. 75% compared to 60% imatinib
C/I: History of coronary artery disease, stroke, periph vascular disease or pancreatitis
SE: 20% cardiac (dose-related), 5% pancreatitis, hyperglycaemia
bosutinib (2nd gen)
More potent than imatinib
Licensed dose is 400mg daily. Starting at 100mg and titrating up over 2-3 months may be better tolerated.
C/I: Nil specific
SE: 30% diarrhoea, transient transaminitis, pancreatitis
Ponatinib (3rd gen)
More potent that 2nd generation TKI’s
Indicated in T315I mutation, which is resistant to most other TKI
Dose 15-45mg daily
Option to reduce to 15mg once BCR-ABL/ABL <1%, majority will continue to respond (OPTIC 2021)
SE: 30% cardiac (likely dose-related risk)
asciminib (BCR-ABL1 STAMP inhibitor)
MOA: BCR-ABL1 STAMP inhibitor, binds to myristoyl pocket.
Approved by NICE for 3rd line use, provided no T315I mutation
ASCEMBL Trial 2021 - 233 patients w/ 2+ prior TKI. 2:1 randomised vs bosutinib
ASC4FIRST 2024 - 405 patients, first line, asciminb vs investigator choice. Earlier MMR and lower rates of discontinuation due to side effects compared to imatinib.
CI: Nil specific
SE: Myelosuppression, Pancreatitis (monitor amylase at start), QT prolongation, Hypertension
TKI-resistant bcr-abl1 mutations
Several resistance mutations have been identified and are indications for switching treatment to a specific TKI known to be active against the given mutation. E.g. Ponatinib for T315I mutation. See table 5 in the ELN guideline or table IV in the BSH guideline for details.
treatment-free remission (TFR)
Many patients will acheive a Deep Molecular Remission (DMR), ie MR4 and MR4.5
How many is many? 50-80% achieve MR4 at 10 years depending on the drug/study
Lots of trial data now supporting discontinuing TKI therapy (summarised in BJH 2018)
Generally after 3 years of treatment, with minimum of 1 year in MR4.5
Majority of molecular relapses occur rapidly, usually at 6-12 months
Loss of deep MR = 51% at 1 year, 54% at 2 years
Loss of MMR = 35% at 1 year, 36% at 2 years
Equals a Treatment-Free Remission of 64% at 2 years
Late loss of MMR beyond 2 years reported in up to 14% of patients
BSH 2020 Criteria for stopping TKI
Discuss case at MDT
Patient should be on a TKI for mimum 3 years, prefarably 5, and should not have
Prior history of advanced phase
Previous TKI resistance
Previously detected BCL-ABL1 KD mutation
Patient should have a typical BCR-ABL1 transcript (ie e13a2 or e14a2) and a MR4 response for the last 2 years
There should be access to a laboratory that can express transcripts to a sensitivity of MR4.5 and a turn around time less than 14 days.
Consider reducing TKI dose by 50% for 12 months prior to discontinuing, with monthly monitoring in that time (see DESTINY trial below)
Monitoring schedule post discontinuation can be found on page 14 of guideline.
ELN 2025 Criteria for stopping TKI
Note: This table remains unchanged in the 2025 guideline
Discontinuation side effects
20-30% of patients report a transient polymyalgia-like syndrome occurring after stopping TKI
Monitoring off treatment (ELN 2025)
PCR every 6-8 weeks for first 6 months
Then every 2 months for months 6-12
Then every 3-6 months thereafter
Re-start treatment at loss of MMR
Re-starting the same TKI at same dose does not prevent patients regaining deep MR (90-95% do so)
Additional data on Stopping TKI Therapy
EURO-SKI 2016
Largest trial so far. 821 patients on 1st line imatinib or after IFN, dasatinib or nilotinib at 1st or more line, excluding patients with prior resistance
Minimum 3 years Rx, with minimum 1 year in MR4
Trial outcome suggests best prognosis is min. 6 yrs treatment, 3yrs in MR4
Definition of molecular relapse was loss of MMR – 45% of patients by 18 months
Outcome: 46% MMR at 12 months
DESTINY 2017
For patients in MR4
Reduced dose for 12 months, and then stopped if patient had not lost MMR
Outcome: 72% TFR rate at 36 months
Conclusions:
Reducing dose is safe (may help with SE’s)
If you stop after prior dose reduction, better chance of staying in MR4 (vs EUROSKI)
For patients in stable DMR after 2+ years of dasatinib.
5 year treatment free remission = 44%
No relapses occurred after 39th month of follow-up
All patients who relapsed and restarted dasatinib regained MMR within 2 months
Other trials – STIM, A-STIM, TWISTER, and others