Essential Thrombocythaemia (ET) (BSH 2010/2014/2021)
50-60% JAK2 V617F
25-30% CALR
3-11% MPL
12-17% ‘Triple Negative’
WHO 2016 Diagnostic Criteria
Requires all major or 1-3 + the minor criteria
Major
Platelet count >450
BM Biopsy
Not meeting criteria for other neoplasm
JAK2, MPL or CALR mutation
Minor
Presence of clonal marker or absence of reactive thrombocytosis
BCSH Diagnostic Criteria
Plt count >450 + JAK2/MPL/CALR mutation + No other myeloid malignancy
BM biopsy not required unless driver mutation not detected
BM Biopsy in ET Diagnosis
Required by WHO but not by BCSH
Good for excluding reactive / other causes and for disease re-assessment over time
Features
Prominent large megas with staghorn nuclei and emperipolesis
Wide spectrum of megakaryocyte morphology present
Reticulin not increased (WHO 0, Bain 1-2)
Significance of JAK2 ET vs CALR ET – No difference in Overall Survival but…
JAK2
Older age
Less high platelet count, but higher Hb and WBC
Increased VTE and transformation to PV
Lower rate of transformation to myelofibrosis
CALR
Younger age
Higher platelet count, but less high Hb and WBC
Reduced VTE and no transformations to PV
Higher rate of transformation to myelofibrosis
Other mutations in ET
High Molecular Risk (HMR) mutations include: SRSF2, SF3B1, U2AF1 and TP53
Associated with poor outcomes, but reduced rates of thrombosis
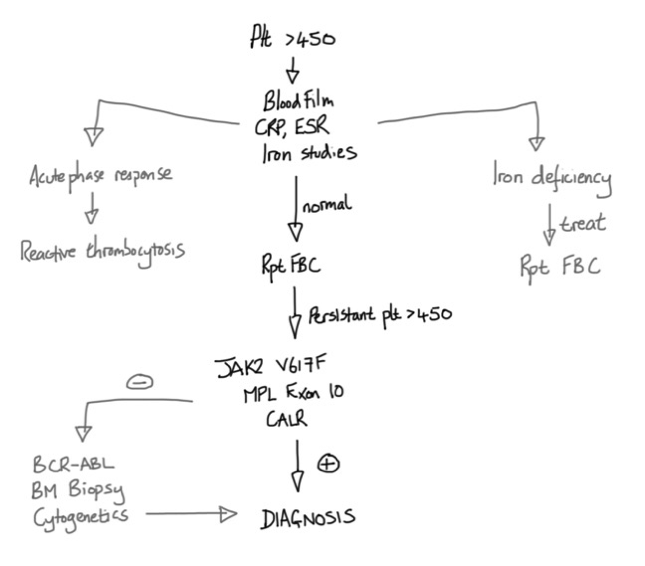
Suggested Investigative Pathway
Prognosis
Life expectancy in first ten years from diagnosis not affected
Morbidity and mortality result from thrombosis, constitutional Sx, MF and AML
Thrombosis risk in ET = 12 per 1000 patient years (greater than MF/AML)
AML transformation – 98% mortality at 3 months without stem cell transplant
Older BCSH Thrombotic Risk Assessment
High risk Age >60 or ET-related thrombosis/haemorrhage or plt count >1500
Intermediate Age 40-60 & no high-risk features
Low Age <40 & no high-risk features
WHO 2012 IPSET-Thrombosis Score
Found no association between thrombosis and platelet count
IPSET Updated in 2015: Taken out CVS risk factors and new categories: V Low, Low, Int, High
--> not used day to day at CUH. Not been linked to management decisions in clinical trials.
Management
All Patients
Aspirin 75mg OD (No prospective trial data. Take age and other factors into account)
(Haematologica 2016: Retrospective study, prone to bias. ?Aspirin not needed for CALR ET)
Manage CVS risk factors – HTN, Cholesterol, Diabetes, Smoking
BCSH Not High-Risk Patients
Only treat if symptomatic or in clinical trial
BCSH High-Risk Patients
Aim for platelet count in the normal range
First line
Second line
Anagrelide – increased risk of MF (need BM biopsy every 3 years)
IFN-Alpha – younger or pregnant patients
Pegylated forms can —> molecular Remission (ASH 2017) but take longer to produce response than HU
(Buslafan, pipobroman, 32P are older alternatives – do increase AML risk)
Notes on PT-1 Trial
High Risk Arm: HU vs Anagrelide, both with aspirin. HU superior. Anagrelide fibrotic risk identified.
High Risk Extended F/up: 75% EFS at 15 years for vascular events. MF transformation more common for MPL patients. All events rare in triple negative patients.
Int. Risk Arm: HU vs no HU, both with aspirin. No benefit to early HU.
Low Risk Arm (unpublished): 250 pts, 73% female, 40% triple negative. Aspirin only. No deaths yet.
Emerging Therapies
mutantCALR monoclonal antibodies - promising early phase results in 2025
ET in Pregnancy
Preconception
Assess risks
Optimise therapy
Contraception - avoid esotrogens (POP/Coil/Depot preferred)
All Patients
Aspirin 75mg OD
LMWH prophylaxis if indicated as per usual pregnancy risk assessment
Uterine Doppler at 20 weeks
Move to high-risk management if abnormal
Avoid dehydration
LMWH for 6 weeks post-partum
High Risk Patients
Interferon
Early LMWH prophylaxis
Regular fetal growth scans
ET in Children
Rare
Different symptoms, thrombosis and natural history compared to adults
Exclude other causes
Aim for conservative approach