Use of FFP & Cryoprecipitate (bsh 2018)
Intro
Fresh Frozen Plasma
Since 2012 there has been a reduction in the use of FFP in UK, but a rise in SD-FFP use
2009 audit – 43% of FFP transfusion given as prophylaxis before procedures (not good)
Cryoprecipitate
Use is increasing, doubled since 2003. Reason is unclear.
Indications
FFP Indications
Correction of multi-factor deficiencies in major haemorrhage / DIC with bleeding
Plasma exchange only when for TTP
Limited role as prophylaxis prior to liver biopsy
Single factor deficiencies if no fractionated product available – i.e. Factor V
FFP NOT Indicated
DIC without bleeding
Warfarin reversal without bleeding
Correcting vit K deficiency in neonates or ITU adults.
Correction of hypovolaemia
Complications
Complications of particular concern with FFP
TRALI (reduced by use of male donors)
Donor anti-A and anti-B
Allergic reactions
vCJD - guidance has changed since 2018, see bottom of page.
Choice of FFP
No difference between FFP recovered from whole blood versus plasmapheresis
If likely to receive multiple repeat transfusions
Consider pathogen-reduced plasma (MB or SD)
Consider Hep A and Hep B vaccination
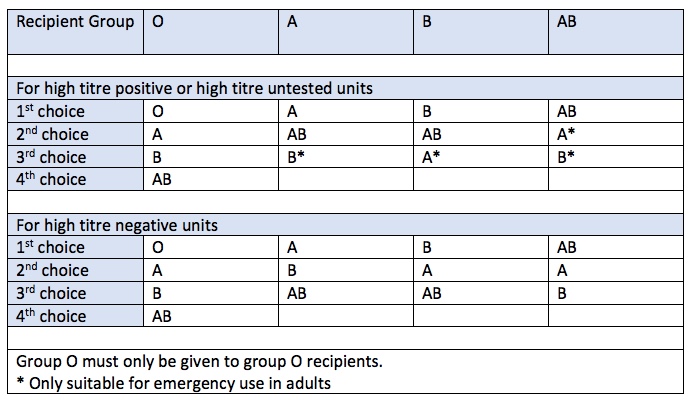
Group O FFP must only be given to Group O patients
A,B,AB patients should receive there own group as first choice, but different group FFP may be used if high-titre negative.
Products of any Rh D group may be transfused, no anti-D prophylaxis is required.
Preparation, Contents & Storage
Fresh Frozen Plasma
180-400ml per pack
From male whole blood or apheresis donors
Not from first time donors
Na 48 mmol/unit, K 1.0 mmol/unit, glucose, calcium (low), citrate, lactate, phosphate
Collection rapidly frozen to -25oC
Plastic packs brittle whilst frozen, handle with care
Can be stored for 36 months at below -25 oC
Factor content requirement
Minimum of 70 IU/ml of FVIII in at least 75% of tested packs
Cryoprecipitate
1 unit = 20-60ml.
1 pool / 1 adult dose = 5 units = 200-280ml
The cryoglobulin fraction obtained from plasma by slow thawing of a single FFP donation at 4 degrees overnight (one unit). This is then re-suspended in small volume of plasma.
FVIII, VWF, FXIII, fibronectin and fibrinogen
Can be stored for 36 months at below -25 oC
Factor content requirement
Minimum of 70 IU/ml of FVIII & 140mg of FGN in at least 75% of tested packs
Pathogen Reduction
All patients born after 1st Jan 1996
Good at reducing levels of enveloped viruses
But not good at reducing non-enveloped viruses (Hep A/E, Parvo). Units tested for these.
MB-FFP (NHSBT) has 30-40% lower FVIII, FXI and fibrinogen activity
SD-FFP (Octaplas) also has lower Protein S and antiplasmin
Thawing FFP
Thawers
Dry heat agitators – limited capacity
Microwave – limited capacity, ?risk of hot spots
Water bath – most common, pack does not come into direct contact with water
Temperature of thawing
33-37oC recommended.
Ranges from 4 – 45oC have been used
Storage after thawing
Fresh Frozen Plasma
Store at 2-4oC
Transfuse within 24 hours of thawing
Transfuse within 4 hours of removal from temp-controlled storage
Pre-thawed plasma can be used up to 120 hours in major trauma.
It can be accepted back into temp-controlled storage on one occasion of <30 min
30 min is based on expert opinion. Further work ongoing. Advice may change.
Purpose of pre-thawing is to reduce delays in major trauma
With exception of protein C, all factors decrease between 24 and 120 hours, FVIII most rapidly and to the greatest extent.
MB-FFP
The shelf-life is not extended beyond 24 hours, due to already lower factor levels.
SD-FFP
Licensed medicinal product and so directed by manufacturer.
Cryoprecipitate
Use immediately once thawed
Store at ambient temperature for maximum of 4 hours
FFP in non-bleeding patients
See also: Bleeding Risk page
British Society of Gastroenterology Guidelines 2020
Strongly recommend against the use of FFP to correct an INR <2.0 prior to liver biopsy, supported by cochrane review 2019
No evidence that it reduces bleeding events
Some evidence that if bleeding does occur, it may be aggravated by increased portal pressures following plasma transfusions.
No requirement for correction of clotting results prior to US-guided paracentesis
Garcia-Tsao 2017 (Variceal bleeds)
American association for the study of liver disease
Recommend against FFP in variceal bleed
May aggravate bleeding through an increase in portal hypertension
PT bore no relevance to bleeding risk in acutely ill cirrhotic patients on ITU
FGN <0.6 and plt <30 were the most important predictors of bleeding
Non-bleeding, critically ill patients with INR 1.5-3.0 about to undergo invasive procedure
Randomised to 12ml/kg FFP or no FFP
FFP only marginal improvement in factor levels
Thrombin generation was unaffected, and anticoagulant levels rose
SABTO 2019 Change to FFP requirement for the ‘96 Club
Previously patients born on or after 1st January 1996 received imported plasma products to reduce the risk of transfusion transmitted vCJD infection. Updated (2019) guidance from SABTO has removed this recommendation following re-assessment of the risks.
There have been no recorded cases of transfusion transmitted vCJD since the introduction of leukodepletion.
Estimated risk of vCJD from UK-plasma is 1 additional vCJD death per in 5.2 million units transfused.