Iron deficiency
iron metabolism
N.B. Red cells contain approx. half of total body iron
Testing for iron deficiency (BSH 2021)
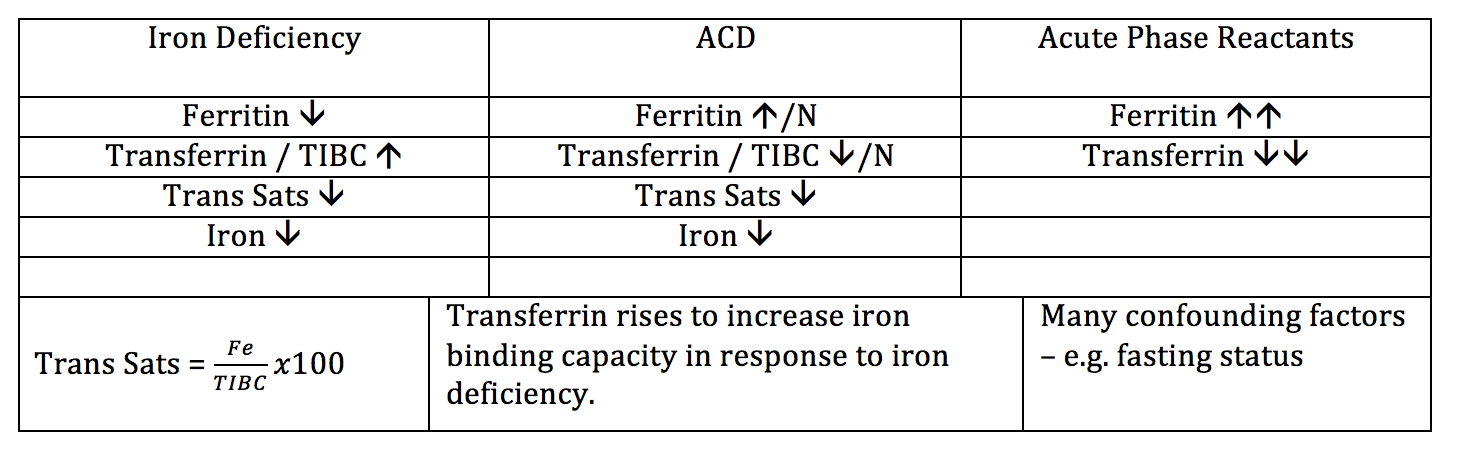
Summary of likely results of Iron Studies in IDA / ACD / Acute Inflammation
Intro
Why is iron deficiency difficult to diagnosis in the laboratory? Look at the diagram above - Iron homeostasis is dynamic and involves many fluctuating parts, e.g. absoprtion, transport, storage, utilisation.
No single test can account for all of these elements.
Recommended starting pointing for the investigation of iron deficiency:
Full blood count + Serum Ferritin + CRP
Serum Ferritin
Ferritin responsible for most protein-bound tissue iron storage. A small proportion of this leaks into plasma —> serum ferritin is a surrogate measure of the intracellular stored ferritin, not of usable circulating iron.
Serum Ferritin 1ug/l = 8mg of stored iron
A normal/raised ferritin is not equivalent to sufficient iron being made available to erythroblasts
BSH 2024 suggested thresholds for likely iron deficiency based on serum ferritin:
<15 = Absolute iron deficiency
<30 = A sensitive+specific threshold of iron deficiency that will respond to treatment
30-100 and Tsat <20% = Possible iron deficiency in presence of inflammation
(WHO uses <70 as a suggested threshold for iron deficiency in presence of inflammation)
Serum ferritin levels >15ug/l may still represent iron deficiency but are less specific when interpreted in isolation. The <15 level is controversial and may change. Many haematologists will treat for iron deficiency at a ferritin <30-50 with other features in keeping with IDA.
Full Blood Count Red Cell Parameters (Hct, MCV, MCH, MCHC, RDW)
FBC confirms anaemia with haemoglobin below reference range
Hb and Hct do not provide any information about iron status
MCV, MCH, MCHC and RDW all potentially low in iron deficiency
MCV normal in up to 40% of iron deficient patients (e.g. consider mixed deficiency)
MCV pre-analytical variables - increases with prolonged storage, and altered by sample temperature
% Hypochromic red cells (%HRC) & Reticulocyte mean Hb content
Methods and acronyms for these tests vary by manufactorer
%HRC
Proportion of RBC with cellular Hb <280g/l and reflects iron status over the last 3 months
>5% suggests iron deficiency
>6% suggests FID in CKD patients who are on ESA’s
Reticulocyte mean Hb content
<29pg suggests iron deficiency
Blood Film
Anisocytosis, microcytosis, hypochromia, elliptocytes, pencil cells, target cells
Also important for suggesting alternative or concurrent diagnoses
Iron Studies
Serum iron
Measures oxidised ferric bound to transferrin
Dynamic level, day-to-day flucutation
It is used to calculate TSAT and should not be interpreted in isolation (some labs will not include in the iron studies report for this reason)
Total iron binding capacity (TIBC) / transferrin
Rise in iron deficiency
Less variable than serum iron but remain non-specific
Transferrin is a negative acute phase reactant
Transferrin saturation (TSAT)
Ratio of serum iron:transferrin/TIBC
% of serum transferrin binding sites occupied by iron molecules
Day-to-day flucuation, poor specificity
<16% supports diagnosis of iron deficiency
Tests not recommended for the diagnosis of iron deficiency
Soluble transferrin receptor (sTfR)
Increased in true iron deficiency. Expensive test, poor availability
Zinc protoporphyrin (ZPP)
Trace byproduct of haem synthesis.
Adds no additional information in simple IDA. Too non-specific to be of use in difficult cases
Potentially used to monitor response to therapy.
Hepcidin-25 Assay
Produced in response to inflammation, high iron —> inihibits iron release from tissues
Suppressed in iron deficiency
Expensive test, unsuitable for high throughput testing
An ELISA is being developed, may have future clinical use
BM aspirate for Pearl’s stain
Provides information on levels of storage iron within the marrow. Should not be performed as a diagnostic test of iron deficiency anaemia.
Iron Supplements
Traditional recommended oral dose = 100-200mg elemental iron/day
Increasing evidence that this is inefficient and likely to increase side effects, as the amount of iron absorbed from any one dose reduces the closer doses are to one another (Due to saturation of absoprtion pathways).
Current practice largely moved to prescribing one tablet per day of chosen preparation, preferably taken in the morning at the same time as a source of vitamin C. Can be reduced to one tablet on alternate days if side effects persist (GI disturbance / constipation). See this RCT from 2020 (Larger trials currently recruiting).
Functional Iron Deficiency / Anaemia of Chronic Disease (FID/ACD) (BSH 2013)
Insufficient iron incorporation into erythroid precursors, despite apparently adequate iron stores (Normal ferritin and BM iron stores).
ACD thought to be intended as a short-term effort to deprive pathogens of free iron, and to allow increased white cell production at the expense of red cells. Becomes a pathological process once occurring chronically.
CKD Patients (NICE 2015)
Serum ferritin is not useful in CKD - elevated in 50% of patients on haemodialysis but not reflective of bioavailable iron. Therefore:
Hb <110g/l + Ferritin 200-800 --> Give IV iron for functional iron deficiency
Hb <110g/l + Ferritin <200 (HD) or <100 (no HD) --> Give IV iron for true iron deficiency
Ferritin >800 --> investigate for iron overload
Iron Deficiency in Pregnancy (BSH 2019)
Adult daily iron requirement = 1-2mg per day
Increases in 3rd trimester = 6mg per day
15% of dietary iron is absorbed, increases to 45% in 3rd trimester
UK – 24% of pregnant women anaemic, up to 46% by time of 28-week check. 14% of non-anaemic women had ferritin <30ug/l in first trimester.
Definitions of anaemia in pregnancy (under r/v by WHO as to validity & practicality, a/w outcome as of 2019):
1st trimester <110g/l
After 1st trimester <105g/l
Postpartum <100g/l
FBC should be tested at booking and 28 weeks (NICE CG62)
Note physiological increase in MCV of approx. 6fl during pregnancy may mask microcytosis
Maternal effects of IDA in pregnancy:
Fatigue
Non-specific symptoms – pallor, weakness, poor concentration, hair loss, headache, palpitations, irritability, dizziness, dyspnea, restless legs, pica.
Poor quality of life, increased risk of post-natal depression
Increased risk of PPH (60% of women with Hb <85 had PPH in a UK prospective observational study)
Fetus/Neonate effects of IDA in pregnancy:
Increased risk of perinatal and neonatal mortality
Increased risk of low birth weight, pre-term birth
?Cut-off for low ferritin in pregnancy?
As ferritin rises along with other acute phase reactants in pregnancy, it may be appropriate to use a higher cut-off than other adults.
But, as of yet, no good research on pregnancy-specific cut-offs of serum ferritin
Current practice is to continue to use <30ug/l as per other adults
Indications for starting empirical oral iron supplementation:
1. If Hb <110 at booking or <105 at 28 weeks
Only check ferritin beforehand if known hbpathy or if planning IV iron
Re-check after 2-3 weeks, Hb should rise by >20g/l
Continue for 3 months and for at least 6 weeks postpartum
2. Non-anaemic women with a high risk of iron depletion (start with or without testing ferritin first)
Previous anaemia
Multiparous (>3)
Multiple pregnancy
Consecutive pregnancies <1 year apart
Vegetarian / Vegan diet
Teenagers
Recent bleeding
High risk of bleeding or Jehovah’s witness
3. As of 2021, the PANDA research programme is developing a planned pilot clinical trial to examine primary prevention of maternal anaemia with use of oral iron (rationale is treating anaemia after it has already developed will not avoid all potential complications)
Indications for testing ferritin prior to starting treatment in non-anaemic women:
High risk of bleeding
Declining blood products, e.g. Jehovah’s witness
Difficulty in providing compatible blood products
Management of Iron Deficiency in Pregnancy
Dietary advice
RDA of iron in 2nd half of pregnancy = 27mg
Haem iron from meat, fish & poultry absorbed 2-3x more readily than non-haem iron
Vit C significantly increase iron absorption from non-haem foods
Tannins reduce iron absorption when consumed with, or shortly after, meals
Once iron-deficient in pregnancy, diet alone is not sufficient to ensure repletion
Oral Iron
Safe, cheap, effective
40-80mg elemental iron once in the morning, or alternate days, on an empty stomach with a glass of water or orange juice.
Higher doses likely to result in increased side effects due to unabsorbed iron
Re-check Hb after 2-3 weeks
Once Hb is in normal range, continue for 3 months or until at least 6 weeks postpartum
IV Iron
Currently recommended for women from the 2nd trimester onwards with confirmed iron deficiency and intolerant / not responding to oral iron. Also consider for women presenting after 34 weeks with Hb <100g/l.
Adv: More likely to achieve target Hb, higher Hb at 4 weeks, and fewer side effects compared to oral iron
C/I: Prev anaphylaxis to IV iron, 1st trimester of pregnancy, active acute or chronic bacteraemia and decompensated liver disease.
Breast feeding: Transient increase in iron in milk, 3 days after treatment, compared to oral iron, but mean concentrations remained within normal range.
Delivery
IDA should not influence mode or timing of delivery
IDA with Hb <100g/l, deliver in an obstetrician-led unit
IDA is an indication for active management of 3rd stage of labour.