intro
Find this useful? You can now tip me on Kofi! Haembase will always be free to access for all but if you would like to support my running costs, and time, this is the place to do it. Thank you!
If possible find a friendly consultant who will mark your answer and provide some feedback. I have tried to give some suggested answers to each question but I am not an examiner and there will be multiple valid approaches I have not considered. Many of them better than what I have written here!
Mock Paper
Paper A Answers - Click to Download - Dr Deepak Chandra, Consultant Haematologist at University Hospitals of North Midlands has provided these thorough model answers to accompany his mock paper (found in the Essay Questions tab). Thank you Dr Chandra!
Individual Questions
question 1
Try to use the first 3-5 minutes to map out the essay before you start, hopefully this way you won’t be crossing out and drawing arrows all over the finished piece.
Whilst reading the question, think about what the examiners are wanting to see in your answer, e.g.:
- They ask for a letter so give them a letter
- Your practical experience/knowledge of preparing a patient for chemotherapy
- Ability to communicate clearly with other healthcare professionals
- Knowledge of the principles for 1st line treatment of Mantle cell lymphoma
- Bonus: Ability to name and discuss relevant clinical trials
Example answer plan:
Dear GP,
Management Plan:
1. 24-hour urine collection for creatinine clearance
2. ECG
3. Bloods inc. FBC, U&E, LFT, Bone, LDH, Hep B, Hep C & HIV
4. Patient information leaflets on Mantle Cell Lymphoma and R-DHAP given to patient
5. Clinic in one week to consent for chemotherapy and book treatment start date
Diagnosis:
Stage 4 Mantle Cell Lymphoma
Performance Status 1
Medications started today:
Allopurinol 300mg OD
Results:
Today’s blood results awaited
Body of letter
- Re-cap patient’s history
- Diagnosis given to patient – mantle cell lymphoma, patient’s presentation typical of the disease, indolent or aggressive course, treatable but not curable, median survival 4-5 years in all comers
- Treatment options – he is a fit patient with stage IV disease and so the goal is chemotherapy followed by autograft, then rituximab maintenance. Treatment is cytarabine-based and a current common regimen is R-Maxi-CHOP/Cytarabine (NORDIC protocol). Bonus: LyMa trial 2017 recently published showing R-DHAP also an effective option. LyMa also demonstrated the benefit of R-maintenance post autograft.
- Risks – neutropenic sepsis (Bonus: mention 24 hr emergency contact card), cytopenias, GI, infusion reactions, renal impairment, iritis, cardiac impairment, tumour lysis syndrome, steroid side effects, hair loss, impaired fertility, treatment-related mortality (The MacMillan website is a great place to look for regimen specific side effects).
- Describe the management plan given above and the rationale for it.
———
question 2
Example answer plan:
Diagnosis
CLL (Binet Stage C).
CLL Score at least 4 (surface Ig not given)
Further work-up:
Consider the need to rule out high grade transformation - is there a site of particular bulk that should be biopsied to ensure still CLL.
TP53 loss / mutation must be looked for (FISH/Molecular) as will direct treatment choices
Patient is anaemic, look for haemolysis - film, reticulocyte count, bilirubin, haptolgobins, DAT
Treatment:
Offer trial
Off trial, assuming no TP53 disruption - chemoimmunotherapy.
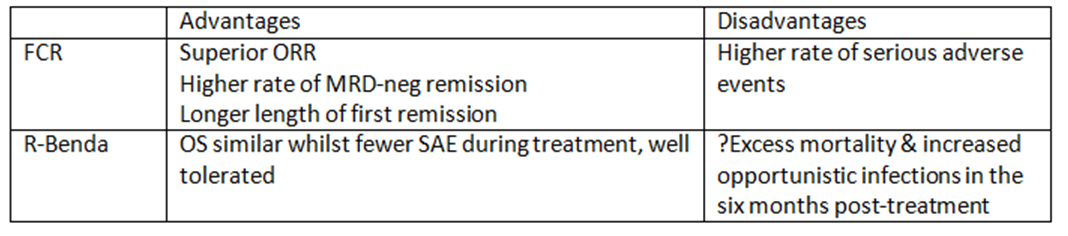
Give your preference and rationale (e.g. FCR based on CLL10 trial)
Supportive Care:
Irradiated blood products for life - purine anologues, bendamustine
consider prophylactic LMWH - is there venous compression 2o to the pelvic disease
Annual ‘flu and pneumococcal vaccination
Patient Support Group,
Relapse:
Re-Stage +/- Re-biopsy
Avoid chemoimmunotherapy as remission only 2 years
Consider ibrutinib or R-idelasilib, based on availability and tailored to patient characteristics.
———-
question 3
Example Answer Plan
Diagnosis:
The probably diagnosis here is TTP
Provide table with a differential diagnosis (other causes of MAHA)
Invesitgation:
Send ADAMTS13 level (<5% diagnostic) but don’t wait for result before proceding with planning treatment
Haemolysis markers - LDH, retic, bilirubin, haptoglobins
DAT - expected to be normal
Troponin - poor prognostic sign if elevated
Coagulation screen - expected to be normal, aids in excluding differentials
Other Tests (for excluding differentials) - HIV, Hep B, Hep C, TFT, Autoantibodies, CT/MRI brain, tumour markers, CT CAP
Management over next 24 hours:
Medical emergency
Multi-disciplinary team-working required
Plasma Exchange with SD-FFP within 4-8 hours.
Removes anitbody + ULMW VWF multimers
Replaces ADAMTS13
SD-FFP reduces risk of TTI and adverse immune responses.
If PEX not available within 4-8 hours, transfuse SD-FFP
Steroids
e.g. 1mg/kg meythlprednisolone
Stops antibody production
Caplacizumab
Anti-VWF antibody, inhibits interactions between VWF and platelets
NEJM 2019 - shorter time to normal platelet count, lower composite mortality
Supportive Care
ITU / high dependency
PPI, folic acid
Red cell transfusion if required
Apheresis Service
Who will need this service (paient population)?
Who will you need on the team?
How will the service be validated / accredited?
What will be needed pre/during/post-procedure for good patient care
See here for some answers to these questions
————
Question 4
Example Answer Plan
A
Immediate Actions:
Stop transfusion, maintain IV access with saline
Assess ABC and call for help - e.g. ITU support / crash team
Check patient identity against blood component being transfused. Examine unit for discolouration / clumps.
Diagnosis:
This is a severe acute transfusion reaction (i.e. Shock is present & this is not in keeping with patient’s underlying condition)
Differential: ABO incompatibility, Anaphylaxis, Bacterial contamination of red cell unit
Investigation:
FBC - ?change in haemoglobin level vs pre-transfusion
U&E - ?acute renal failure
LFT/Bilirubin - ?haemolysis
PT/APTT/FGN - ?DIC
Group & Screen - for comparison to previous samples
Direct antigloublin test
Blood cultures
Urine - ?haemoglobinuria
Return blood unit to transfusion lab for confirmation of typing / in case of further testing for bacterial contamination
Chest X-ray - ?ARDS/Pul oedema/TACO/TRALI
(For review at later date:
Tryptase, IgA level, haptoglobins)
Further management:
?ABO incompatibility - Intensive supportive care, dialysis, involvement of renal team
?Anaphylaxis - IM adrenaline 0.5ml of 1:1000
?Bacterial Contamination - broad spectrum IV antibiotics, contact NHSBT to consider component recall
B
Root Cause Analysis
Consider all steps in the ‘vein-to-vein’ pathway (2005 Blood Safety and Quality Regulations)
Patient ID minimum requirements
Documentation - prescription, request form
Sample handling & labelling
Laboratory systems - electronic data management, lab analysers
SHOT report 2017
1 ABO-incompatible red cell transfusion, 342 near misses, majority wrong blood in tube
Issues around ABO-incompatibility formed 2 of the 3 key recommendations for that year
Corrective Action
Re-sample patient
Duty of candour to patient to explain why re-testing
Preventative Action
Staff education
?Need for new work flows/systems in the day unit
Two sample policy - this case an example of its utility.
External Reporting
Report to SHOT haemovigilance scheme
———-
Question 5
A
Delayed haemolytic transfusion reaction
Repeat group and screen with reference to pre-transfusion sample
FBC, Reticulocyte count, U&E, Bilirubin, LDH, haptoglobins, DAT, Clotting Screen, Urinalysis
Transfuse for symptoms, with red cells negative for offending antigen if identified
Report incident to SHOT
B
Lab – compulsory antibody identification for each subsequent transfusion episode
Clinical – delayed transfusion due to testing and/or availability, acute haemolysis, DHTR, HDFN for female patients
Bonus – include some SHOT stats. E.g. For three years running (2015-2017), SHOT has reported deaths definitely related to transfusion due to delayed provision of blood.
C
Pre-compatibility testing – e.g. 3 day sample validity if transfused in last 3 mo.
Those at increased risk get Rh and Kell matched blood – e.g. Sickle cell
National programme for red cell genotyping in sickle cell (no longer free)
RhD negative units for RhD negative women of child bearing age
RAADP scheme for RhD negative pregnant women
Question 6
The diagnosis is ITP.
Differentials, investigations and treatment options can be found here.
In our practice we would not recommend a bone marrow biopsy in this case.
There is no single protocol for the treatment of ITP and the question hints at the patient having some medical background so the answer reflect the weighing of advantages and disadvantages of treatment options.
Make sure you stated clearly what your preferred treatment would be (no single right answer, but the question wants you to get off the fence).
A pregnancy management plan for ITP can be found here.
Question 7
A
Physical examination
Bloods - inc Hep B serology, Cholesterol, lipase, HbA1c - to address CVS risk factors and TKI choice
ECG - TKI’s prolong QTc, identify cardiac pathology
BM Biopsy
Aspirate - ?Disease Phase - % Blasts, % Basophils
Trephine - Degree of fibrosis (prognostics)
Cytogenetics - To identify Philadelphia chromosome & Additional Chromosomal Abnormalities (ACA) in Ph+ cells
Treatment Options
Imatinib - fewer serious side effects, generic available, slower time to DMR
Nilotinib - more potent than imatinib, coronary artery disease, pancreatitis
Dasatinib - more potent than imatinib, pleural complications 5% per year, pul HTN, opportunistic infections, platelet function defect
B
Fortnightly FBC until haematological remission, then:
C
STIM1 (Stop Imatinib 1) was proof of concept - 38% still in remission after 77 months off treatment
DISCONTINUATION SIDE EFFECTS
20-30% of patients report a transient polymyalgia-like syndrome occurring after stopping TKI
MONITORING OFF TREATMENT
Monthly PCR for first 6 months, then 8 weekly for next 6 months
Then every 3 months after the first year
RE-START TREATMENT AT LOSS OF MMR
Re-starting the same TKI at same dose does not prevent patients regaining deep MR (90-95% do so)
Question 8
A
Diagnosis is DLBCL. Not double hit or Burkitt as no MYC rearrangement present.
Performance status 1 - restricted from strenuous activity (ie full time job on feet) but otherwise independent)
Stage 4 disease
IPI Score 2 - for LDH and Stage
Standard of care would be R-CHOP-21 x6 with interim PET-CT to assess response
Make sure to cover supportive care - gastroprotection, infection prophylaxis, bone protection
Additional bloods prior to chemo - Hep B, Hep C, HIV, EBV serology
B
Don’t forget to follow style guideline (ie clinic letter)
CNS-IPI 3 - for LDH, Stage and kidney involvement. Not high risk score (4-6)
But CNS prophylaxis recommended on basis of kidney involvement
Risk of CNS relapse 2-6% for all comers with DLBCL. Could be approaching 10% for this patient.
Offer HD-MTX - would need to ensure renal function adequate (abnormal at presentation)
Current debate - to intercalate or give at end of R-CHOP
More about DLBCL and CNS prophylaxis here.
Question 9
A
Screen for end organ damage
Aggressive iron chelation preconception reduces end-organ damage
Diabetes common in b-Thal – HbA1c <43 mmol/mol for 3 months prior to conception
TFT – ensure euthyroid
Cardiac – ECG, Echo and T2* MRI
Liver iron – Ferriscan, ideally liver iron should be <7mg per gram of dry weight
Abdo USS – gallstones, liver cirrhosis, hepatitis
Bone density – Offer bone density scan and ensure vitamin D replaced
Vaccination
Hep B vaccine (+ Hib and pneumococcus if hyposplenic)Pen V prophylaxis if hyposplenic
Review Medication
Folic acid 5mg daily
Discontinue teratogenic medicationsStop deferiprone and deferasirox 3 months prior to conception
Avoid desferrioxamine in 1st trimester. Can be used safely at low doses after 20 weeks
Stop bisphosphonates 3 months prior to conception
Haemoglobinopathy screening
Screen partner
Offer IVF/ICSI with pre-implantation genetic diagnosis to avoid homozygous / compound heterozygous pregnancies if both partners have significant hbpathy.
B
Schedule
Monthly review to 28 weeks then fortnightly
Monthly HbA1c if diabetes
Cardiac assessment at 28 weeks if b-Thal Major
Scans
Offer viability scan at 7-9 weeks
Routine 1st trimester scan at 11-14 weeks
Routine anomaly scan at 20 weeks
Additional monthly growth scans from 24 weeks
Transfusion
If b-Thal Major , regular transfusion to maintain pre-transfusion Hb >100g/l
b-Thal Intermedia, start transfusion if worsening maternal anaemia or growth restriction
Thromboprophylaxis
Splenectomy or Plt >600 – Aspirin
Splenectomy & Plt >600 – Aspirin + LMWH
Delivery plan (Don’t forget the style guide, i.e. short letter to another doctor):
If known red cell antibodies, crossmatch blood for delivery in advance
Intrapartum fetal heart rate monitoring is recommended (increased risk of fetal distress)
b-Thal Major – desferrioxamine should be infused during labour
Active management of third stage of labour to reduce blood loss
Based on the Green Top guidelines, summarised here
question 10
A
This is transfusion-associated cardiac overload (TACO).
Other, less likely, differentials includes transfusion-associated dyspnoe (TAD), transfusion-related lung injury (TRALI), ABO incompatibility and symptoms due to underlying medical condition rather than the transfusion.
Stop transfusion, ABCD assessment of patient, check patient ID against donor units, examine units for clumps/discolouration, treat pulmonary oedema (diuretics etc), call for help / escalate level of care if required.
B
Transfusion process can be broken into 10 steps, with potential for errors to occur at each stage.
Decision to transfuse and consent patient
Request
Sample taking
Sample & request receipt
Testing
Component selection
Component labelling
Component collection
Prescription/Authorisation
Administration
Positive patient identification critical at steps 1, 3 and 10
Steps 4-7 are critical laboratory steps
See SHOT reports more detail and useful summary diagrams.
C
Consider using Corrective Action, Preventative Action (CAPA) or Root Cause Analysis (RCA) formats to structure your answer.
Some issues to consider:
Legal requirement to report to MHRA (BSQR is the UK responsible body)
Professional responsibility to report to SHOT haemovigilance scheme (also a lab accreditation and hospital quality assurance scheme requirement).
Local reporting to Hospital Transfusion Team will aid in the above and allow for local intervention and audit.
Education/training to consider whether patient clinical state is consistent with a single laboratory result
Give One and Review schemes
Question 11
A
Using an example, issues include:
Estimated risk of a particular procedure causing bleeding
Ease of controlling any bleeding that occurs
Potential consequences of any bleeding that occurs
B
Current issues - e.g. infection, anaemia
Focused bleeding history - e.g. bruising, epistaxis, menorrhagia, prev. haemostatic challenges
Past medical history - e.g. renal impairment, portal hypertension
Drug history - e.g. Anticoagulants, antiplatelets
Examination - e.g. bruising, bleeding, signs of liver/renal disease, joint hypermobility
Bloods - focused testing based on outcome of the above
C
Remember to give examples
Delays surgery
Anxiety for patients
False reassurance to surgeon
Expensive and uses up lab time
Poor predictor of bleeding risk
E.g. Some APTT reagents only sensitive to FVIII <30 iu/dL —> will miss mild haem A / VWD
PT/APTT may be normal in patients taking direct oral anticoagulants
Or result maybe abnormal in a healthy patient
E.g. FXII deficiency, Lupus Anticoagulant
Question 12
A
Acute Myeloid leukaemia.
U&E, LFT, Coag, HIV, Hep B/C, HLA typing
Bone marrow biopsy - aspirate morphology, karytoyping, FISH and molecular genetics
See AML page for ELN genetic risk stratification. E.g. RUNX1 favoruable, Monosomy 7 Adverse
B
Assess presence of frailty, ideally using comprehensive geriatic assessment (GA) can aid decision making / sometimes prognosis. GA includes comorbidity, cognition, mental health, functional status, frailty, nutrition, polypharmacy, social support, quality of life.
MDT input to cover the above - phsyio, OT, elderly care medicine, palliative care
Examples for tools to make these assessments include the Hospital Anxiety and Depression (HAD score)
Examples of tests include ECG, Echocardiogram
C
Standard induction chemotherapy
Venetoclax-Azactidine
Azacitidine monotherepy (Blasts <30%)
Low dose Cytarabine
Supportive care alone (+/- Hydroxycarbamide)
See AML page for details of adv/disadvantages
D
E.g. Venetoclax - Inhibitor of BCL2. BCL2 is a anti-apoptotic protein that is overexpressed in cells of many haematological malignancies. Apoptosis is reduced in these cells because BCL2 sequesters the pro-apoptotic protein BAX. —> Inihibiting BCL2 releases BAX which leads to cell death.
Question 13
A
Tumour type - highest risk cancers (adjusted for prevalence) are pancreas, ovary and brain
Age, Immobilisation, Surgery, CVC lines, infection, chemotherapy, radiotherapy
B
Minimum 3 months and then continue for as long as line in situ
LMWH - QoL implications
DOAC - drug interactions, body weight, renal function, intraluminal tumours, limited evidence specific to line thrombosis
Warfarin - no. Inadequate for cancer thrombosis
C
Aim to keep line in, provided:
Still functioning
Correctly positioned
No evidence of infection
Symptoms from the VTE resolve
Minimum AC prior to line removal?
If must be removed, ISTH 2014 guidance statement recommends 3-5 days AC prior to removal, if clinically practical. There is expert opinion only. Othe guidelines make no recommendations.
Since then, Blood Advances 2021 —> retrospective review of >600 pts. Early removal of CVC, with or without AC, was not associated with an increased risk of PE. Will this change future recomendations?
D
There is no randomised trial evidecne to direct treatment for CAT occurring with thrombocytopenia
ISTH 2018 Guidance for first 30 days of CAT with platelet count <50:
High risk - transfuse platelets to a plt count >40-50 and give full dose AC
Low risk - adapt AC dose to platelet count (e.g. 50% dose if plt 25-50, omit <25)
(High risk = includes, but not limited to, symptomatic PE, proximal DVT, previous VTE)
(Low risk = line-associated VTE, asymptomatic subsegmental PE), distal DVT)
Consider IVC filter only in patients with absolute contraindication to anticoagulation.
Blood 2022 - post hoc analysis of Hokusai trial. Review 100 pts with plt <100. 2x increased risk of bleeding complications from anticoagulation. (only 14 pts had plt count <50)
CAVEaT 2022 - UK audit found variable adherence to ISTH guideline.